 |
Current statistics show that only 3% of physicians (healthcare providers) in clinical practice have participated in clinical trials.1 Have you ever considered participating in clinical research? Some of us have taken a close look at whether it might add to our busy day by getting involved in doing research. Some clinical practices have already participated in different forms of research. There’s a full range of options available, such as marketing research and FDA pre-market approval studies in contact lenses and pharmaceuticals to collaborative data collection that might be funded by the National Institutes of Health.
For those in clinical practice who might be interested in research and haven’t yet taken the plunge, let’s take a close look at some considerations that might help you. Specifically, why should one get involved? I’ll attempt to answer this question in this issue, and, in the next, I’ll share some ideas on how to best get involved if you’re committed to getting started.
Motivations for Pursuing Research Projects
Here are some insights I think optometrists should take advantage of if they seize this opportunity:
1. Expanded access to new products, testing capabilities and care.1,2 If your patients are eligible, they will have access to new treatment options and receive additional attention and close follow-up often without additional cost.1-3 Contact lens and pharmaceutical manufacturers are often interested in gathering additional information from a marketing perspective, and this is a great entry point for eyecare providers.
2. Advancing innovations through clinical trials.2,4 You might have the opportunity to move a new product or drug successfully through the regulatory pipeline prior to approval or help develop additional research questions to answer a specific question that might aid in innovation.
3. Boosting your own knowledge/staying up to date with developing technology.1 Clinicians will often comment about much they glean from the information gathered during clinical studies. Discussing results and ideas with contemporaries can also be quite invigorating and sometimes stimulate new clinical questions that might have value with added research.
4. Increased clinical trial patient retention rates with higher patient satisfaction scores supporting your practice reputation.2,3 When your patients know you’re striving to stay abreast of the newest information and that your practice is willing to take the time to advance knowledge, it boosts their confidence in you and your practice. In addition, they generally receive additional time and attention (more frequent tests and visits).2
5. Potential for additional financial compensation.1,2,4 This definitely helps add to the bottom line of your practice at the end of the year.
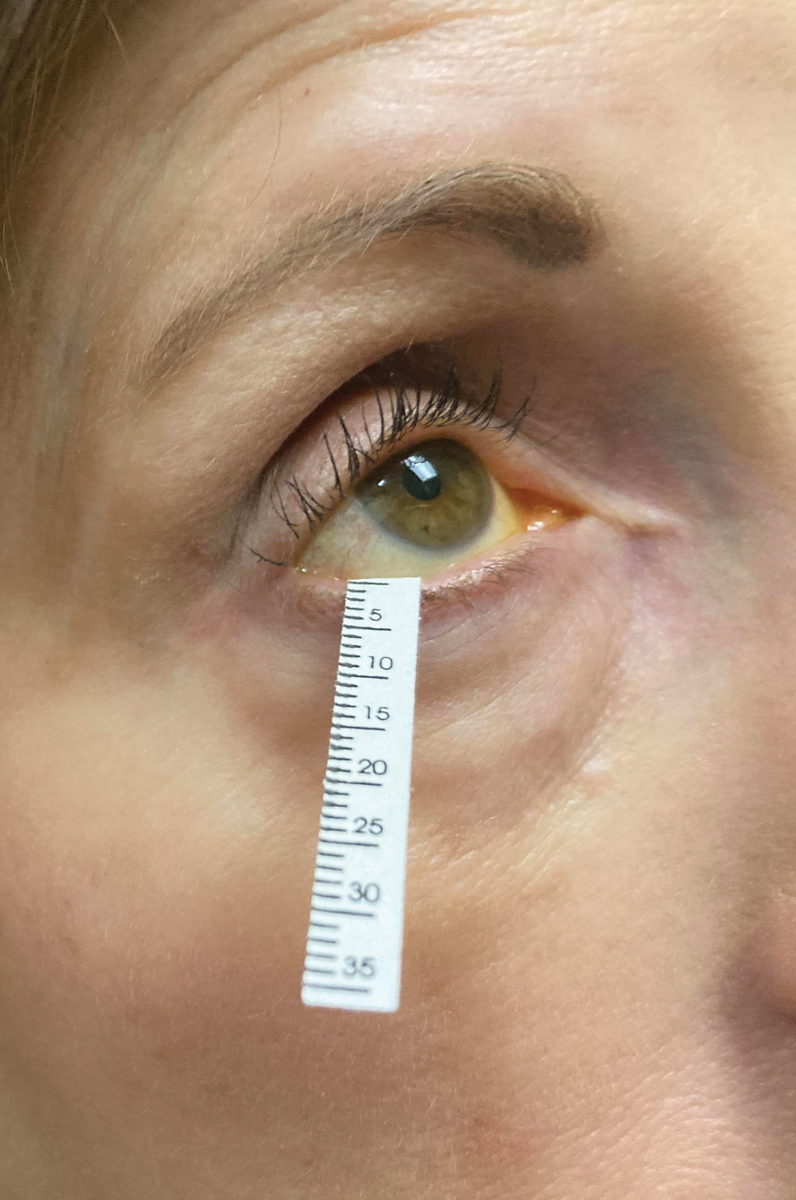 |
|
Schirmer’s strips are still an often used mainstay of clinical trials for dry eye drug performance. Photo: Jessica Steen, OD. Click image to enlarge. |
Consider taking on one of the avenues to do research in your practice. You won’t be sorry that you do. I find studies intellectually stimulating and provide a variety of work experiences. What really makes it rewarding for me is when research ultimately improves patient care with previously unmet needs for our patients.
Stay connected! So, come up with a thought-provoking question to get started and look for the right team partner.
In the next issue, we’ll look at some additional ideas on how to get started in clinical research.
1. Lazas D. Five reasons physicians should integrate clinical trials into their practice. Applied Clinical Trials. www.appliedclinicaltrialsonline.com/view/5-reasons-physicians-should-integrate-clinical-trials-into-their-practice. July 31, 2023. Accessed August 1, 2024. 2. Lazas D. How integrating clinical trials into private practice can benefit both patients and physicians. Pharmacy Times. www.pharmacytimes.com/view/how-integrating-clinical-trials-into-private-practice-can-benefit-both-patients-physicians. May 9, 2023. Accessed August 1, 2024. 3. Obrenović Ž. Doing research in practice. Research & Practice. 2014;20(4):15-7. 4. How to get started in clinical trials. Retina Times. 2022;40(1):93. |


