Blink Mechanics: Why it Matters
This incredibly important function has huge implications for dry eye and contact lens wear, especially when it goes awry.
By Marc-Matthias Schulze, PhD, Dipl. Ing. (AO)
 |
Release Date: February 15, 2020
Expiration Date: February 15, 2023
Estimated time to complete activity: 2 hours
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group.
Educational Objectives: After completing this activity, the participant should be better able to:
- Discuss how the blink works, and what happens when it doesn’t work right.
- Review how reduced blink rates brought on by digital device use impact ocular health.
- Describe how the blink affects contact lens wear.
- Assess a patient’s blink clinically.
- Identify what treatment options are available.
Target Audience: This activity is intended for optometrists engaged in the care of patients with corneal dystrophies.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Review Education Group. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education, and the American Nurses Credentialing Center, to provide continuing education for the healthcare team. Postgraduate Institute for Medicine is accredited by COPE to provide continuing education to optometrists.
Faculty/Editorial Board: Marc-Matthias Schulze, PhD, Dipl. Ing. (AO), the Centre for Ocular Research & Education (CORE)
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 66365-AS. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Dr. Schulze has no disclosures.
Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
A blink is the rapid closing and opening motion of the eyelids, and may be either involuntary or voluntary.1,2 But is blinking really this simple? What if it isn’t performed properly or frequently enough? How does this affect your patients with dry eye disease (DED) or contact lens (CL) wearers complaining of discomfort and dryness?
This article reviews blink mechanics and how to assess them in the clinic and discusses how blinks can go wrong and what this means for your patients. It also reviews the options your patients have to improve their blink routine.
How Does a Blink Work?
Involuntary blinks can be sub-divided into spontaneous and reflex blinks. Spontaneous blinks occur subconsciously, are triggered by the autonomic nervous system and account for the majority of the blinks performed during the day.3 Spontaneous blinking helps to maintain an intact pre-corneal tear film to ensure optical quality as well as proper re-wetting of the ocular surface, and helps with removal of tear film debris.1,4,5
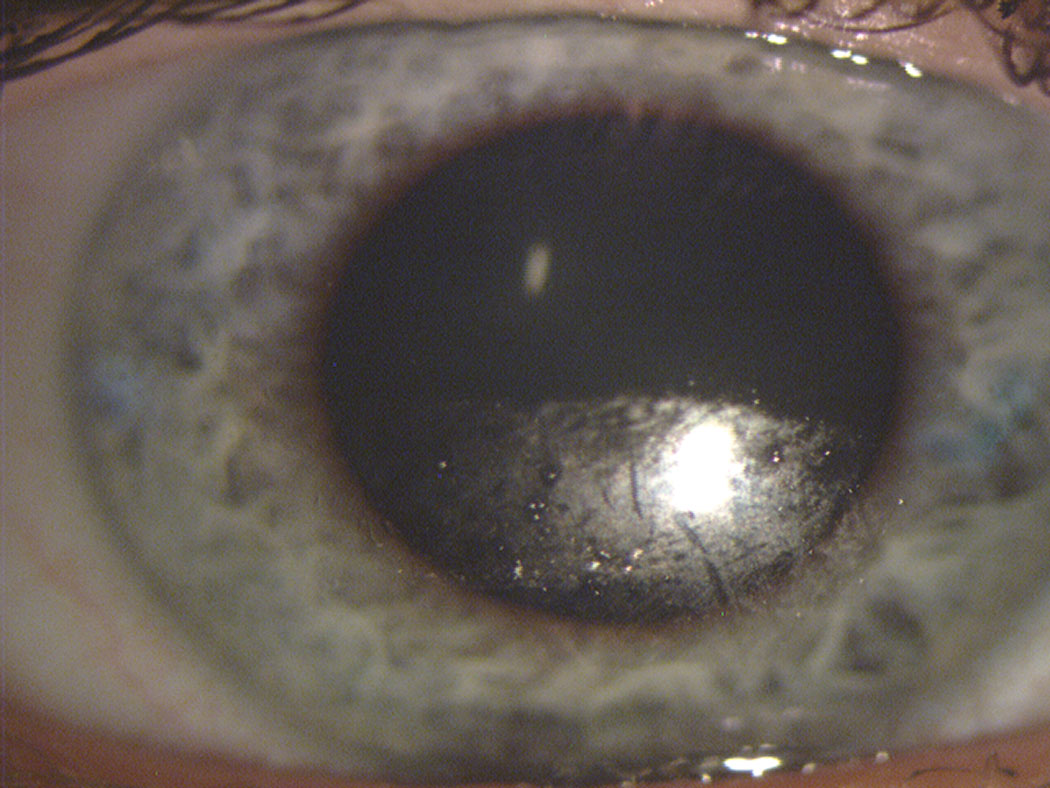 |
| Fig. 1. This contact lens surface shows substantial drying across the inferior portion after a partial blink. Note the clear transition from dark (replenished) superior to speckled (dried) inferior portion of the lens, identifying where the downward movement of the blink ended. Photo: The Centre for Ocular Research & Education (CORE). Click image to enlarge. |
Reflex blinks are typically triggered by some kind of external stimulus, such as a sudden bright light, a loud noise or a foreign body touching the anterior surface of the eye.1 Voluntary or consciously performed blinks are intentional lid movements for a specific purpose, for example to achieve re-wetting of the ocular surface after prolonged eye opening. Voluntary blinking is typically used during blinking exercises that aim at altering a patient’s blink habits for the better.5
The blinking process involves movements of both upper and lower lid, with the upper lid doing the primary closing and opening motion, while the lower lid only exhibits a minimal upwards and nasal movement.6 During a full eye closure, the upper eyelid touches the lower eyelid in the temporal location first, followed by a wave-like swiping motion nasally that also assists in tear exchange and drainage at the lacrimal puncta of the eyelids.1,4,6
Opening and closing of the eyelids is controlled by innervation of the 3rd and 7th cranial nerves, respectively, which innervate the levator palpebrae superioris and orbicularis oculi muscle, respectively.2 Innervation of the levator palpebrae superioris muscle as well as Mueller’s muscle contributes to raising the eyelid and holding it up, while the orbicularis oculi muscle closes the lids.6,7 During a blink, the nervous system turns off the tonically active levator palpebrae superioris, allowing the orbicularis oculi muscle to rapidly lower the upper eyelid before the levator palpebrae superioris becomes active again and raises the lid.6
Complete eye closure is a crucial requirement for ocular surface health, as the contact of upper and lower lid during a complete blink promotes secretion of lipids from the meibomian glands, which protect the tear film from evaporation.5 During a complete blink, mucins are secreted from the tarsal goblet cells over the entire ocular surface and tear film; for incomplete blinks, this only applies to the areas covered by the moving eyelids, thus impacting tear film integrity.5
The presence of incomplete spontaneous blinks is therefore of concern, particularly in patients with evaporative DED.5,8,9 Researchers recently reported a two-fold increased risk for developing DED in patients with incomplete blinking patterns, suggesting it may be a predisposing factor towards the eventual development of evaporative DED.8 Compared with participants with complete blinking patterns, participants who exhibited incomplete blinks were found to have significantly worse symptoms and signs of DED, including greater ocular surface disease index (OSDI) scores, greater meibomian gland drop-out and poorer meibum quality, as well as reduced lipid layer thickness and tear film stability.8,10
Blink Rate Specifics
A blink is typically subdivided into four parts: the downward motion, the turning point, the upward motion and the inter-blink period.9,11 With completeness of a blink being the most important factor when it comes to blink mechanics, the inter-blink interval requires equally significant clinical attention as it relates to blink frequency. The inter-blink interval is inversely related to the blink rate—the longer the inter-blink intervals (i.e., the time between each consecutive blink), the fewer blinks a patient performs per minute.
Based on an extensive review, the average number of blinks per minute was reported to typically range from about eight to 22 blinks per minute in normal human subjects under different conditions, with large inter-subject differences reported in the literature even while performing the same tasks.1,5,12 Various factors can impact blink rates, including gaze (lower in reading posture vs. conversational primary gaze), computer use (reduced) or certain medications such as birth control pills (32% more blinks/minute in women taking birth control pills).12-19 Studies suggest patients with medical conditions such as anxiety or panic disorders have higher blink rates, while patients with Parkinson’s disease blinked less frequently than normal controls.20,21
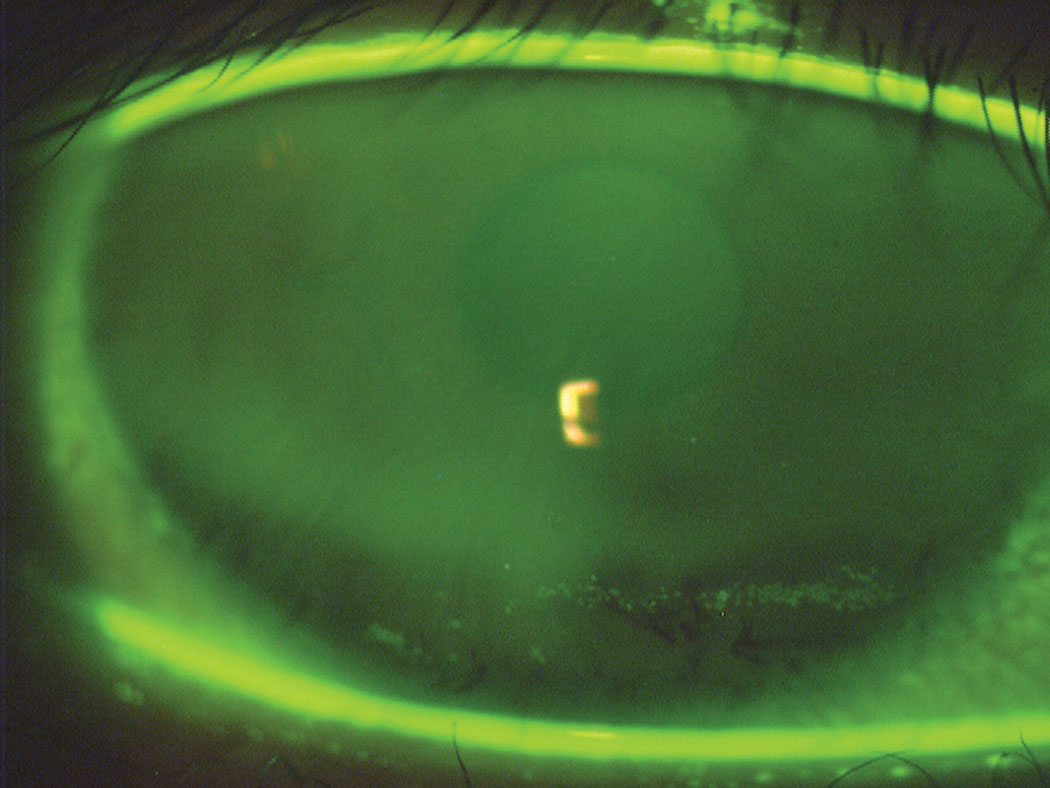 |
| Fig. 2. This image depicts inferior dehydration staining due to incomplete blinking. The darker horizontal band in the stained area corresponds to the turning point of the lid between the downward and upward motion of the blink. Photo: The Centre for Ocular Research & Education (CORE). Click image to enlarge. |
Not surprisingly, dry eye patients were found to have significantly higher blink rates than asymptomatic controls.14,16 This was particularly the case for patients with evaporative DED, who typically have rather short tear break-up time (TBUT) due to their compromised lipid layer and are strongly affected by prolonged inter-blink intervals. To avoid symptoms or any potential ocular surface damage, a dry eye patient’s inter-blink interval needs to be shorter than their TBUT; in other words, they need to blink before their tear film breaks up.1,5
Blink rates vary depending on the required level of attention, with significantly more blinks during tasks that require less attention, such as watching a movie or during conversations, compared with more difficult tasks such as playing a video game.14,15,22 The more cognitively demanding task of reading resulted in reduced blink rates compared with the simpler baseline task of observing a landscape picture, independent of the text being read on a computer screen at 100% or 330% display size, on a tablet, or when reading a hardcopy of the same text in silence or aloud.23 Interestingly, the same study also shows that reading on an electronic screen results in a significantly higher percentage of incomplete blinks than when reading the paper hardcopy.23
Simply put, when a task requires a lot of attention, we tend to stare for longer periods of time, resulting in a thinning of the tear film and eventual tear film break-up. Many of the tasks surrounding digital device use, however—be it the computer at work or an addictive game on a smartphone—do require the attention levels that result in insufficient blink rates and dryness symptoms.
Keeping in mind that blink rates in normal subjects typically range from eight to 22 blinks per minute, imagine a dry eye patient with a TBUT of about two seconds while using a computer, trying to focus on a task on their screen. To avoid dry eye symptoms and to maintain a completely and continuously replenished ocular surface in this scenario, the patient would theoretically need to perform more than 30 complete blinks per minute, much exceeding the typical blink rate of up to 22 blinks.
The impact of digital device use on ocular dryness is not only a concern in adult but also in pediatric patients. Studies show that the increased use of digital devices in school, coupled with the child’s own smartphone or tablet use, is associated with increased symptoms and signs of dry eye that are reduced when smartphone use is stopped for four weeks.24,25 In a group of 99 children between the ages of four and 17, 42% were found to have some level of meibomian gland atrophy, highlighting the importance of evaluating DED in pediatric patients.26
Blinking and CL Wear
Contact lens wear and blinking are closely linked, with both blink mechanism and frequency impacting contact lens wear. Conversely, CL wear directly affects blink mechanics and frequency.1
Studies show that blink rate increased significantly after study participants were fitted with CLs compared with when no lenses were worn.27,28 However, changes in blink rate from lens wear are thought to be temporary only, and to return to normal rates after CL wear.1 In a study during which contact lens wearers were asked to perform four different digital device tasks (watching a Ted Talk, playing a word search game, playing a Tetris-like game and reading Wikipedia articles), blink rates during CL wear were between 1.7 to 2.5 times higher than when the same tasks were performed while wearing spectacles.22
| Blink Exercises for the Busy Patient Performing blink exercises at regular intervals on a continued basis is crucial to achieve improvements, but many times, patients forget about their blinking exercises due to busy schedules. To help patients remember, clinicians can recommend a number of free tools, ranging from general break reminders combined with blink exercises to more specific blink exercise tools. Here are a few options: • http://eyeleo.com (Windows only). While not necessarily blink exercise-specific, this small piece of software provides PC users with regular pop-up reminders to perform short (eight seconds; modifiable) eye exercises to prevent eye strain and improve ocular comfort by closing the eyes, blinking or looking from side to side. Preferences regarding frequency can be set up easily and include other break options such as “locking” the screen for a few minutes for an actual break away from the desk. • www.regularbreaks.com (Browser based). This simple and free tool runs in any web browser in the background and provides pop-up reminders for regular breaks. Individualized break schedules can be set up, or the tool can be used for conscious blink exercises, as well as a reminder to drink (more) water or to get up for a stretch away from the desk. • Donald Korb Blink Training (iOS app). This offers recorded verbal instructions on how to properly pace lid movements when performing the blink exercises and has a built-in reminder option; however, in its current version, there is a limit of three reminder notifications every 30 minutes or every hour before a new set of reminders has to be manually triggered. • Blink Blink (Windows only).38 This is a computer-based blink-animation software that operates in the background. It mimics the blink movement of upper and lower eyelid by introducing two partially transparent bars onto a user’s computer screen that simultaneously move in from top and bottom and then apart again. In a clinical study, computer users were found to have increased blink frequency after using the application for one week. A copy of the software can be obtained free of charge from the study author.38 Beyond these free options, additional fee-based smartphone or computer apps are available in the applicable app stores. |
Contact lens wear is known to disrupt tear film integrity, with a measurable worsening of tear film quality during the inter-blink interval when contact lenses are in place.29 During CL wear, there are smaller amounts of pre-lens tear film compared with the pre-ocular tear film, which is also thinner than when no lenses are worn; CL wear also frequently results in a compromised lipid layer.1,5,8 As a consequence, TBUT or the development of dry spots on the CL surface occur significantly earlier compared with no lens wear, especially in patients reporting symptoms of discomfort compared with asymptomatic lens wearers (Figure 1).1,30
Vice versa, poor blinking habits can negatively impact CL wear.1 Insufficient blinking, often during digital device use, can lead to the drying of the contact lens surface, affecting optical quality as well as causing discomfort.1,5 Incomplete or twitch blinks (minimal movements of the upper lid) result in areas of de-wetting in the inferior portion of the contact lens, affecting comfort and potentially reducing optical quality if an incomplete blink fails to replenish the tear film in front of the pupil.1,5 Incomplete blinking can make lenses more prone to deposits, as evident from lens surface deposits in the inferior region of non-rotating, prism-ballast toric contact lenses that showed no deposits in the superior lens regions that were re-wetted by the partial blink.1,5
To effectively counteract these CL wear complications, patients with symptoms of dry eye and discomfort must ensure that their inter-blink interval is constantly shorter than the pre-lens TBUT.
Blink Assessment
The clinical assessment of each patient’s blink mechanism should be a routine component when evaluating patients with DED or those complaining of discomfort or dryness during CL wear. Depending on available chair time and severity of symptoms, those assessments may include basic metrics such as counting the number of blinks per minute (blink rate) or metrics potentially associated with or caused by a patient’s blinking action such as tear film quality or TBUT.
Questionnaires and patient lifestyle. With the ever-increasing prevalence of digital devices in everyone’s lives, collecting information about hours of digital device use as well as lifestyle habits, both for the work place and at home, should be a routine component of every eye exam. Additional tools such as symptom questionnaires—such as the OSDI, standard patient evaluation of dye dryness (SPEED), 5-item dry eye questionnaire (DEQ-5) and 8-item contact lens and dry eye questionnaire (CLDEQ-8)—may also provide valuable insight into potential causes and triggers of the dry eye symptoms.10,31-33 The CLDEQ-8 specifically addresses questions for CL wearers, including the patient’s need to close their eyes during lens wear.33
Blink mechanism. Blink rate is perhaps the most obvious assessment of a patient’s blink mechanism and is typically assessed by counting how often a patient blinks per minute. However, as simple as this appears, it may still involve potential pitfalls for the clinician. With blink rates being highly dependent on task difficulty, only a few scenarios can be assessed in the exam room. It is also crucial that patients are not aware that the blink is being assessed, as this can induce voluntary or forceful blinking.8,15,34 Thus, the brief, casual conversation you have with your patient at the beginning of an eye exam may be used for a mental count of blinks to get a general idea about your patient’s blink habits.
Incomplete blinking is perhaps best assessed at the slit lamp biomicroscope, after instillation of sodium fluorescein. While assessing the ocular surface, observe whether any dark horizontal bands parallel to and above the lower lid margin appear after the patient blinks. The presence of these dark lines indicates that there is incomplete eye closure and that the turning point between downward and upward motion of the blink is above the lower lid, leaving the inferior portion of the eye exposed without tear film replenishment (Figure 2). Similarly, superficial epithelial staining in the same area (also referred to as exposure staining) is a sign of continuous incomplete blinking habits.
The availability of imaging instruments such as the LipiView II (Johnson & Johnson Vision) also allows for a non-obvious assessment of blink rate and completeness. As a side-product of its lipid layer examination feature, this tool provides an automated analysis of the number of blinks performed during the 20-second lipid layer thickness measurement, which are reported as partial (i.e., incomplete) and total blinks (Figure 3).
Tear film break-up time. According to the Tear Film and Ocular Surface Society’s Dry Eye Workshop II (DEWS II) report, TBUTs of <10 seconds in at least one eye are indicative of evaporative DED.35 Shorter TBUT has been associated with incomplete and too infrequent blinking that may be indicative of evaporative DED and is a simple but valuable assessment in clinical practice. TBUT is best assessed noninvasively, ideally with a device that projects placido disc-based ring patterns onto the tear film, such as the Keratograph 5M (Oculus) or the E300 corneal topographer (Medmont). Patients are asked to blink twice, and the time until a change or disruption of the reflected ring patterns is measured, either automatically or manually using a stopwatch.
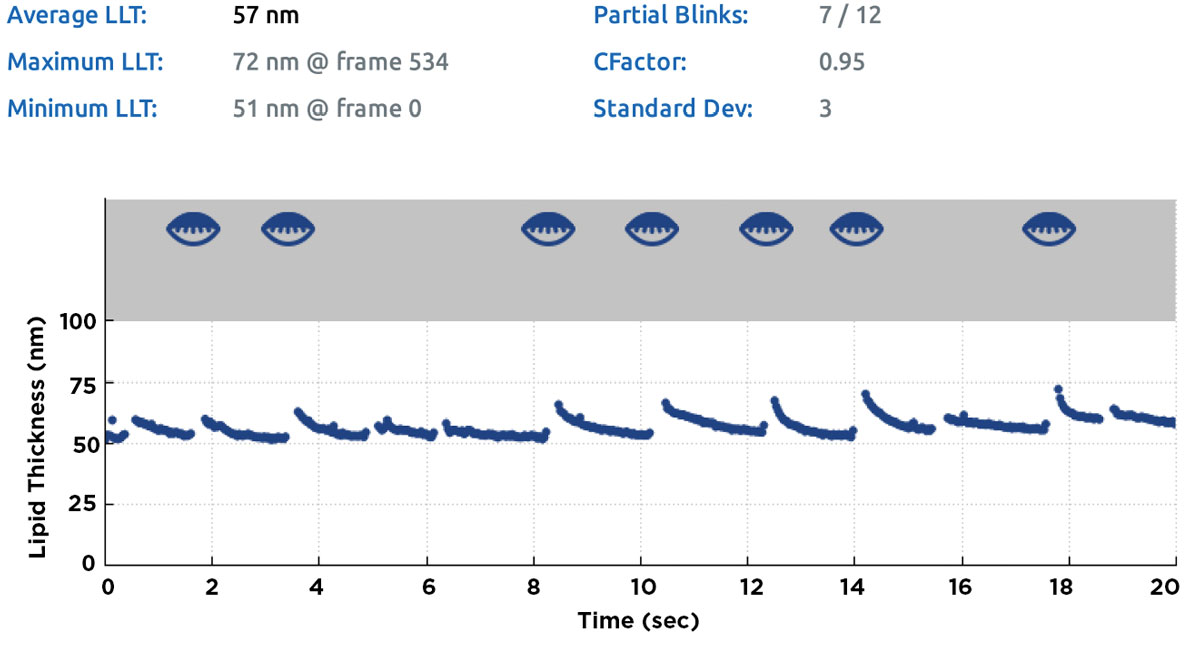 |
| Fig. 3. Summary screen of a lipid layer thickness measurement obtained with the LipiView II. Partial blinks during the 20-second measurement and corresponding time-points are identified as partially closed lid icons in the grey area of the chart; the count for partial/total blinks (in this case, 7/12) is given in the summary table above the chart. Click image to enlarge. |
Assessments of TBUT following sodium fluorescein instillation using blue light and a yellow filter are options if noninvasive testing is not available, although this typically resulting in shorter TBUT compared with noninvasive testing.35 Both pre-corneal as well as pre-lens TBUT are valuable metrics for clinical settings to evaluate a patient’s tear film over time.
Meibomian gland assessment. For patients who do not blink fully, the expression of meibum from the meibomian glands is reduced, eventually causing gland blockage and atrophy.5 Assessment of the meibomian gland structure using devices such as the Keratograph 5M or the LipiView II allow for quick visualization of the glands, in addition to monitoring changes over time. Meibography images are highly suitable for patient education, as patients can see the current status of their glands first-hand. Meiboscopy is a clinical technique during which the meibomian glands are assessed at the slit lamp using transillumination of the lids.36 The assessment of meibum quality, using tools such as the meibomian gland evaluator, provides information whether additional treatments (e.g., applying heat) to improve meibum quality may benefit your patient (Figure 4).37
For those interested in further assessment options, the DEWS II Diagnostic Methodology report provides a thorough review of diagnostic measurement options for DED that go beyond the blink-related assessments presented here.35
Treatment Options
Dry eye patients, contact lens wearers and anyone spending significant amounts of of time in front of a computer screen can benefit from blink exercises that introduce a more frequent as well as consistent blink routine throughout the day.34,38 Most blink exercises focus on conscious blinking to achieve complete eye closure, with frequent repetitions recommended so that full blinks become the habitual blinking mechanism.39
The most well-known exercise, particularly for those spending extended periods of time at their desk each day, is probably the 20-20-20 rule—every 20 minutes, take a 20-second break to look at a target at least 20 feet away.40 Although conscious blinking is not mentioned in this rule, the simple action of looking away from the screen is typically accompanied by blinking and helps replenish the ocular surface.
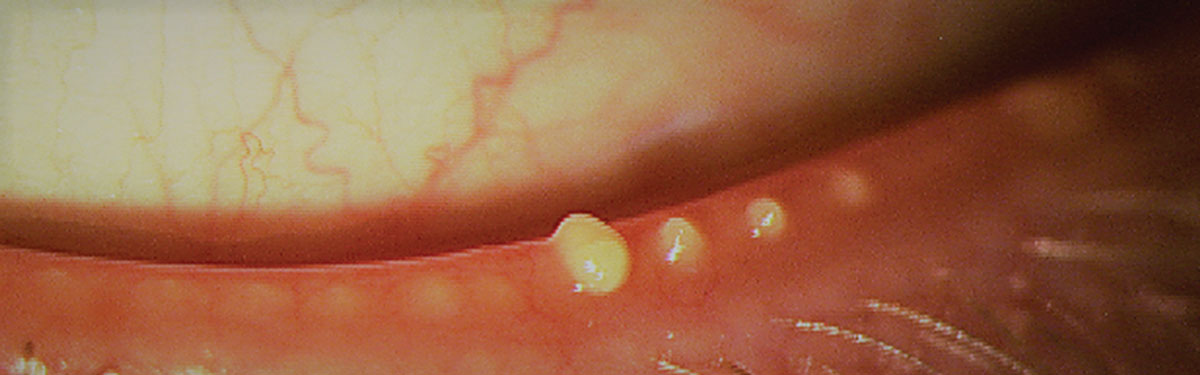 |
| Fig. 4. Blocked meibomian glands after manual expression. The cloudy appearance suggests poor meibum quality. Photo: The Centre for Ocular Research & Education (CORE). Click image to enlarge. |
A number of specific blinking exercise resources exist. During a common exercise patients are asked to complete two different blinking sequences, recommended to be repeated every 10 to 12 minutes, to improve their habitual blinking mechanisms.39 In the first exercise, patients are asked to close their eyes normally, pause for two seconds, and open their eyes again. This is directly followed by the second exercise, during which patients also close their eyes normally and pause for two seconds, but additionally squeeze their lids forcefully for two more seconds prior to opening their eyes.
Squeezing the eyelids helps develop the muscles involved in eyelid closure and trains them to be use in the habitual blinking process.39 To test whether the blink is performed properly, patients are asked to place their fingers at the corner of their eyes and blink. If they feel any movement, the defense muscles rather than blinking muscles are used.39 The squeezing of the eyelids overemphasizes complete closure and muscle contraction.39
Reminding your patients of the importance of proper blinking habits should be a routine recommendation to all of your patients, but specifically for those where poor blink mechanics such as incomplete or infrequent blinking are suspected or observed. Patients who spend prolonged periods of time using digital devices are also more prone to poor blinking habits and potential ocular surface drying due to reduced blinking frequency.
Blink exercises are highly useful tools that any affected patients should introduce into their daily routines to achieve and then maintain regular and complete blinking patterns. These should assist in better ensuring continuous tear replenishment, result in a higher likelihood of meibum expression and improved ocular surface health.
Dr. Schulze is a senior clinical scientist at the Centre for Ocular Research & Education (CORE) at the School of Optometry and Vision Science, University of Waterloo, ON, Canada, where he is responsible for conducting clinical studies involving contact lenses, dry eye, the anterior segment and ocular imaging.
1. Efron N. Blinking abnormalities. In: Contact lens complications. Edinburgh: Elsevier; 2012:39-46. 2. Bologna M, Agostino R, Gregori B, et al. Voluntary, spontaneous and reflex blinking in patients with clinically probable progressive supranuclear palsy. Brain. 2008;132:502-10. 3. Korb DR, Blackie CA, McNally EN. Evidence suggesting that the keratinized portions of the upper and lower lid margins do not make complete contact during deliberate blinking. Cornea. 2013;32:491-5. 4. Pult H, Riede-Pult BH, Murphy PJ. A new perspective on spontaneous blinks. Ophthalmology. 2013;120:1086-91. 5. McMonnies CW. Incomplete blinking: exposure keratopathy, lid wiper epitheliopathy, dry eye, refractive surgery, and dry contact lenses. Cont Lens Anterior Eye. 2007;30:37-51. 6. Gordon G. Observations upon the movements of the eyelids. Br J Ophthalmol. 1951;35:339-51. 7. Evinger C, Manning KA, Sibony PA. Eyelid movements. Mechanisms and normal data. Invest Ophthalmol Vis Sci. 1991;32:387-400. 8. Wang MTM, Tien L, Han A, et al. Impact of blinking on ocular surface and tear film parameters. Ocul Surf. 2018;16:424-9. 9. Jie Y, Sella R, Feng J, et al. Evaluation of incomplete blinking as a measurement of dry eye disease. Ocul Surf. 2019;17:440-6. 10. Walt J, Rowe M, Stern K. Evaluating the functional impact of dry eye: the Ocular Surface Disease Index. Drug Inf J. 1997;31:b5. 11. Braun RJ, King-Smith PE, Begley CG, et al. Dynamics and function of the tear film in relation to the blink cycle. Prog Retin Eye Res. 2015;45:132-64. 12. Doughty MJ. Consideration of three types of spontaneous eyeblink activity in normal humans: during reading and video display terminal use, in primary gaze, and while in conversation. Optom Vis Sci. 2001;78:712-25. 13. Acosta MC, Gallar J, Belmonte C. The influence of eye solutions on blinking and ocular comfort at rest and during work at video display terminals. Exper Eye Res. 1999;68:663-9. 14. Himebaugh NL, Begley CG, Bradley A, Wilkinson JA. Blinking and tear break-up during four visual tasks. Optom Vis Sci. 2009;86:106-14. 15. Jansen ME, Begley CG, Himebaugh NH, Port NL. Effect of contact lens wear and a near task on tear film break-up. Optom Vis Sci. 2010;87:350-7. 16. Ousler G, Abelson MB, Johnston PR, et al. Blink patterns and lid-contact times in dry-eye and normal subjects Clin Ophthalmol. 2014;8:869-74. 17. Patel S, Henderson R, Bradley L, et al. Effect of visual display unit use on blink rate and tear stability. Optom Vis Sci. 1991;68:888-92. 18. Schlote T, Kadner G, Freudenthaler N. Marked reduction and distinct patterns of eye blinking in patients with moderately dry eyes during video display terminal use. Graef Arch Clin Exp Ophthalmol. 2004;242:306-12. 19. Yolton DP, Yolton RL, Lopez R, et al. The effects of gender and birth control pill use on spontaneous blink rates. J Am Optom Assoc. 1994;65:763-70. 20. Karson CN, Burns RS, LeWitt PA, et al. Blink rates and disorders of movement. Neurology. 1984;34:677-78. 21. Kojima M, Shioiri T, Hosoki T, et al. Blink rate variability in patients with panic disorder: New trial using audiovisual stimulation. Psychiatry and Clinical Neurosciences. 2002;56:545-9. 22. Schulze M, Wong A, Haider S, et al. Blink rate in silicone hydrogel contact lens wearers during digital device use. Optom Vis Sci. 2016;93:165122. 23. Argilés M, Cardona G, Pérez-Cabré E, Rodríguez M. Blink rate and incomplete blinks in six different controlled hard-copy and electronic reading conditions. Invest Ophthalmol Vis Sci. 2015;56:6679-85. 24. Moon JH, Lee MY, Moon NJ. Association between video display terminal use and dry eye disease in school children. J Pediatr Ophthalmol Strabismus. 2014;51:87-92. 25. Moon JH, Kim KW, Moon NJ. Smartphone use is a risk factor for pediatric dry eye disease according to region and age: a case control study. BMC Ophthalmol. 2016;16:188. 26. Gupta PK, Stevens MN, Kashyap N, Priestley Y. Prevalence of meibomian gland atrophy in a pediatric population. Cornea. 2018;37:426-30. 27. Hill RM, Carney LG. The effect of hard lens wear on blinking behaviour. International Contact Lens Clinic. 1984;11:242-8. 28. Carney LG, Hill RM. Variations in blinking bahaviour during soft lens wear. International Contact Lens Clinic. 1984;11:250-3. 29. Alonso-Caneiro D, Iskander DR, Collins MJ. Tear film surface quality with soft contact lenses using dynamic-area high-speed videokeratoscopy. Eye Contact Lens. 2009;35:227-31. 30. Rohit A, Willcox MDP, Brown SHJ, et al. Clinical and biochemical tear lipid parameters in contact lens wearers. Optom Vis Sci. 2014;91:1384-90. 31. Keir N, Ngo W, Situ P, et al. Evaluation of the standard patient evaluation of eye dryness (SPEED) questionnaire. Invest Ophthalmol Vis Sci. 2013;54:6028. 32. Chalmers RL, Begley CG, Caffery B. Validation of the 5-Item dry eye questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Ant Eye. 2010;33:55-60. 33. Chalmers RL, Begley CG, Moody K, et al. Contact lens dry eye questionnaire-8 (CLDEQ-8) and opinion of contact lens performance. Optom Vis Sci. 2012;89:1435-42. 34. Collins M, Heron H, Larsen R, et al. Blinking patterns in soft contact lens wearers can be altered with training. Am J Optom Physiol Opt. 1987;64:100-3. 35. Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15:539-74. 36. Robin JB, Jester JV, Nobe J, et al. In vivo transillumination biomicroscopy and photography of meibomian gland dysfunction: a clinical study. Ophthalmology. 1985;92:1423-6. 37. Korb DR, Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea. 2008;27:1142-7. 38. Nosch DS, Foppa C, Toth M, et al. Blink animation software to improve blinking and dry eye symptoms. Optom Vis Sci. 2015;92:e310-5. 39. TearWell. Blinking Exercises. TearWell Advcanced Dry Eye Treatment Center; Southern College of Optometry. www.speakcdn.com/assets/2519/tw_blinkingexercises.pdf. Accessed October 9, 2019. 40. American Optometric Association. Computer Vision Syndrome. www.aoa.org/patients-and-public/caring-for-your-vision/protecting-your-vision/computer-vision-syndrome?sso=y. Accessed October 17, 2019. |


