As advances occur within the field of specialty contact lenses, their utility expands to encompass wider purposes. Originally posed as a corrective measure for irregular corneas, scleral contact lenses have quickly found use for refractive error correction, including high astigmatism, keratoconus and other ectatic corneas, post-surgical eyes and eyes suffering from significant dryness from any underlying disease process. With a wider patient base that could potentially benefit from scleral lenses, optimizing the fitting process outcomes becomes an important goal.
 |
|
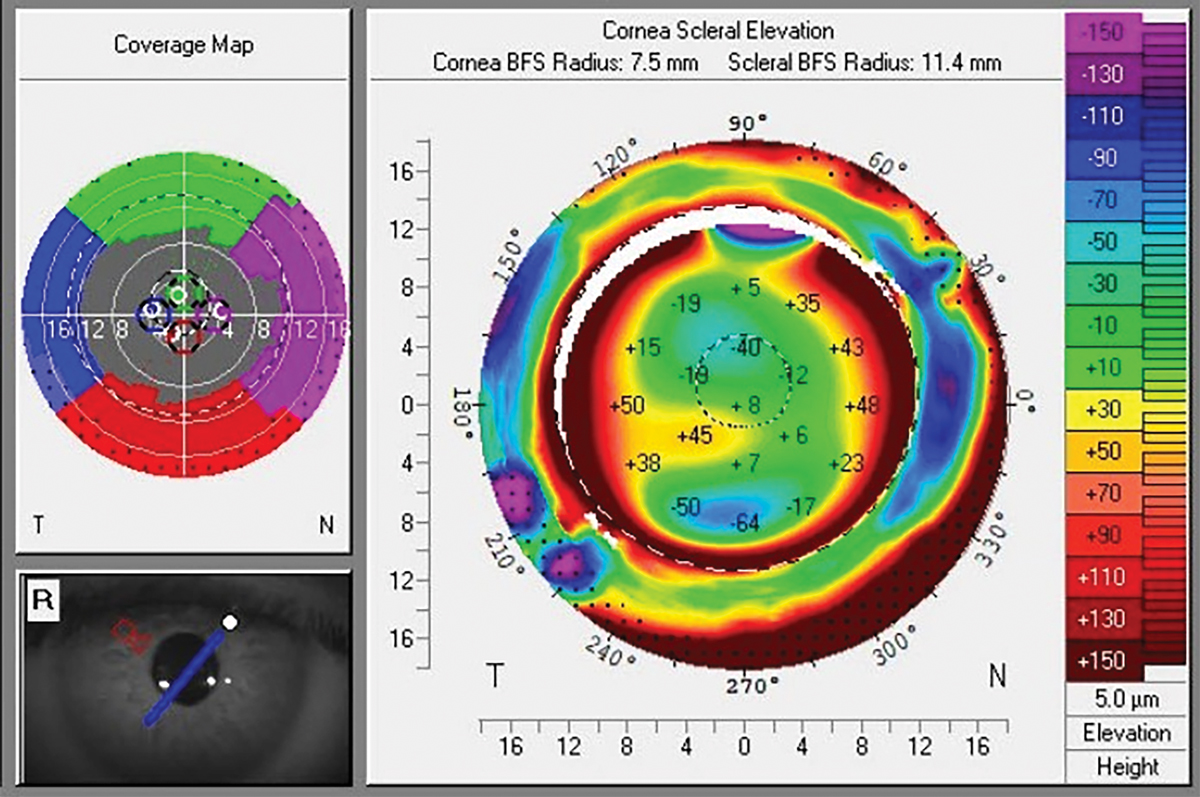
Fig. 1. CSP scan of keratoconic eye with marked scleral asymmetry, note scleral principal meridians do not follow corneal toricity. Click image to enlarge. |
Fitting and Design
Technology advancements in scleral lens design are promoting efficiency in the fitting process and increasing customization capabilities. Any scleral lens fitter is familiar with the multiple-visit process that specialty lenses typically require, which involves various tweaks on prescription, sagittal depth, limbal clearance and peripheral landing zones. With the advent of profilometry devices, scleral lenses could be designed from anterior surface imaging, such instruments include Eaglet, Cornea Scleral Profile (CSP) on Pentacam and similar. With these technologies, elevational differences of sclera vs. corneal toricity are more easily assessed, as compared with using diagnostic lenses and evaluating lens settling after a period of 30 to 45 minutes.1 A recent study looked into a data provided by 423 practitioners who fit scleral lenses for keratoconus comparing image-based vs. impression-based technology. Practitioners with image-based technology used it for 25% of lens fittings, those with impression-based technology used it for 9% of fittings and those with both types of technology used image-based devices for 23% of fittings and impression-based for 8% of fittings.2
The benefit of corneoscleral profilometry is the ability to determine, without using a diagnostic lens, the necessity for a spherical, toric or quadrant-specific edge design, as well as the potential need for a microvault (Figure 1).3 These technologies have opened new possibilities for efficiently achieving optimal scleral lens fit, even in patients with extreme variations in surface elevation. Dual-elevational scleral lens technology is being adopted by a greater number of practitioners to incorporate advanced designs into their clinical practice. One of the studies presented at the last Global Specialty Lens Symposium 2024 meeting looked at predicting dual-elevation scleral lens rotation. The average dual sagittal depth difference was 263µm, and a difference of 200µm to 300µm was achieved in 87.5% of eyes. Average rotation was 19.7º with these lenses.4 Another poster compared the sagittal depth measurements of seven different clinically available instruments in one subject. The Medmont, sMap3D, Visante and Cylite measurements were found to be within 60µm of each other through four of five chords. Some instruments measured consistently higher or lower values than others.5
New Depths
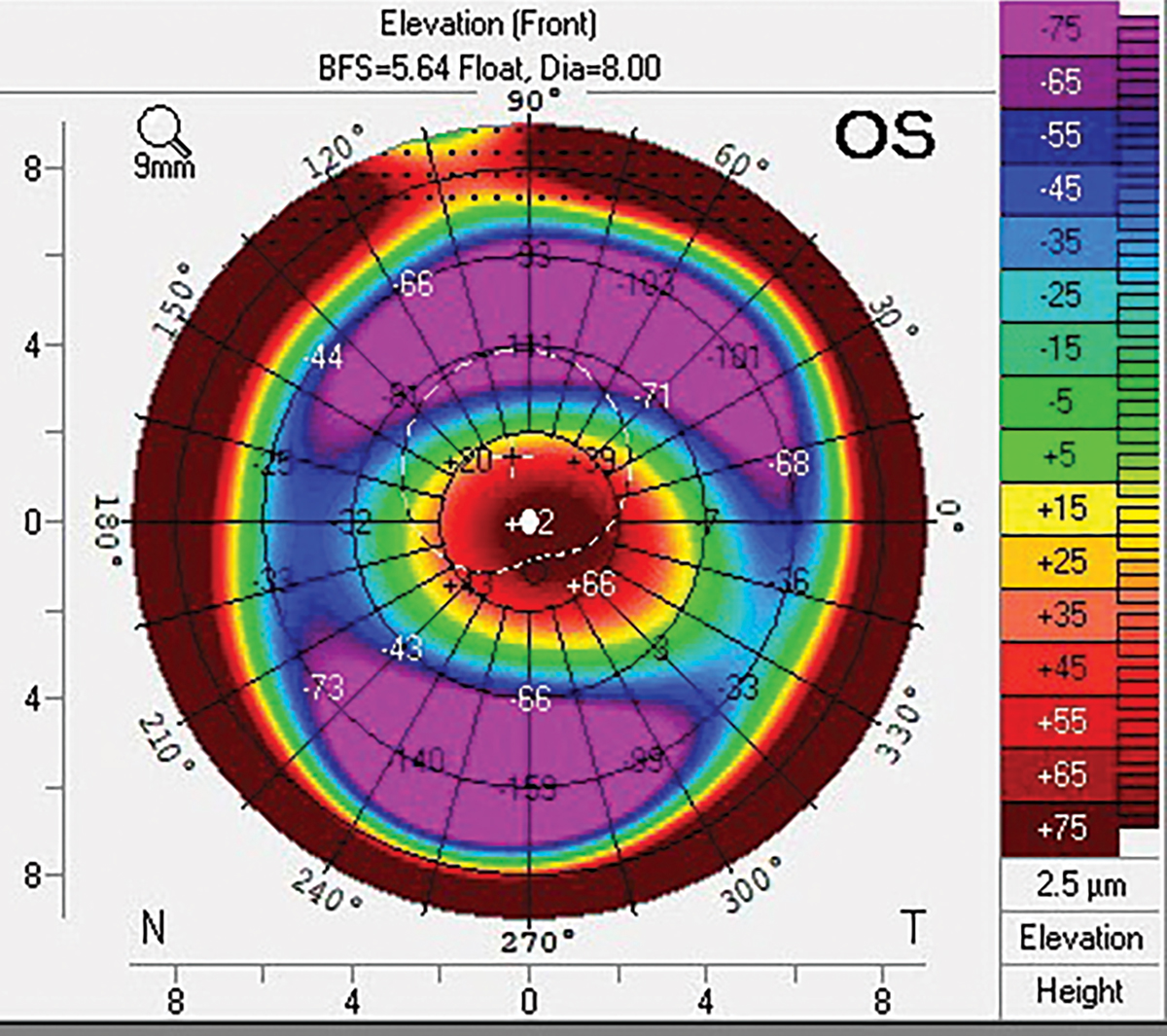
Dual-sagittal depth and quad-sagittal depth scleral lenses, such as the Bi-elevation and Echo lens designs from Bausch + Lomb allow for further customizations to increase stability and optimize fit. Eyes with significant corneal toricity and elevation differences, such as post-trauma or with advanced keratoconus, are not optimally fit with a standard scleral lens design where a single sagittal height separates the lens from the corneal surface (Figure 2). An ideal chamber depth for a scleral lens vaults around 200µm to 250µm across the entire cornea post-settling, which is an important balance of reducing suction pressure vs. avoiding cornea-lens touch, while maximizing visual quality.6 These customizations allow for even distribution of post-lens tear film (vault) across the entire cornea, promoting stability of the lens and reducing debris accumulation within the tear chamber and optimizing visual acuity.7
 |
|
Fig. 2. Pentacam front elevation scan of keratoconic cornea with marked elevational differences along horizontal and vertical meridians. Click image to enlarge. |
Quad-sagittal depth lens design (Echo) was developed on principles of its predecessor, the Bi-elevation lens, by allowing quadrant-specific sagittal height adjustment and rotation of the peripheral landing zone of the lens to correct a mismatch in corneal versus scleral elevation and toricity, therefore improving lens chamber centration over the cornea and providing better scleral alignment. The amount of landing zone rotation against the corneal chamber could be extrapolated from profilometer imaging, and the amount of elevational differences along principle corneal elevational meridians could be implemented when designing an initial trial scleral lens.
Tackling Problems
Considering the progress that has been made with scleral lens technology warrants inquiry into the challenges being addressed.
A common issue faced by many scleral lens wearers is mid-day fogging, which is the accumulation of cellular debris, mucin and the natural tear film components within the fluid reservoir.8 Anywhere from 26% to 46% of scleral lens wearers suffer from mid-day fogging, which results in blurry vision, discomfort and the need to remove and refill the scleral lenses, sometimes multiple times per day.8 With extensive research being conducted on the contents of fogging, there is the possibility of pro-inflammatory markers contributing to the debris due to unbuffered or unbalanced pH saline surface toxicity and mechanical impact to the corneal and limbal epithelial cells.9,10
Suggested modifications to the scleral lens to avoid this debris include proper alignment of the lens haptics to the peripheral sclera 360º, avoiding excessive limbal vault or contact and reducing central clearance of the lens.11 Adding a viscous, preservative-free solution within the lens, such as Refresh Celluvisc (Allergan), can reduce the tear exchange to eliminate the need to repeatedly refresh the lens solution, or at a minimum prolongs wear time.11
An additional challenge faced by specialty lens fitters is higher-order aberrations and their degradation to a patient’s optical quality. A cornea with high irregularities, whether from surface elevation, scarring or other causes, will have increased aberrations, which lead to halos, ghosting and overall reduced quality of vision.12 Newer developments to reduce these aberrations include wavefront-customized scleral contact lenses, which map out an aberration profile in these irregular eyes which can then be used to design a scleral lens that neutralizes those aberrations to an acceptable level.13
Other scleral lens designs incorporate various levels of higher order aberration control, which can be used diagnostically with patients to determine the best subjective endpoint (BostonSight). For patients who have already achieved an ideal fit but still subjectively suffer from poor vision, higher order aberration control should be considered.
Uses for Dry Eye
Applications of scleral lenses discussed thus far have mainly covered refractive error and irregular corneas, but these lenses also offer significant therapeutic benefits. Patients who are immunocompromised, undergoing oncological treatments, suffering from autoimmune conditions or on multiple systemic medications typically will have some degree of dry eyes.14,15 Dryness may present as aqueous-deficient, where there is not enough liquid within the tear film, evaporative, where there isn’t enough oil being produced to prevent the tears from evaporating, or inflammatory, where cytokines and other inflammatory markers continue a cycle of inflammation as a response to an irritated surface.14
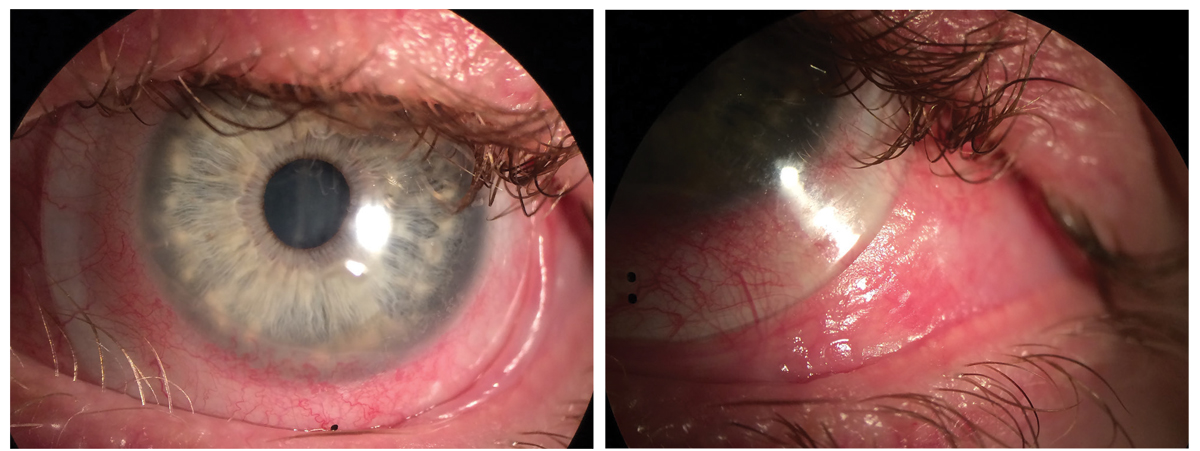
Often, surface involvement is multifactorial and has some degree of all these components, such as in cases of ocular cicatricial pemphigoid (OCP; Figure 3). This rare autoimmune, sight-threatening disorder affects women twice as much as men. Corneal and conjunctival cicatrization from ocular surface inflammation and compromised lid anatomy, along with decreased tear production, may lead to corneal opacification, ulceration, perforation and devastating vision loss. To address these debilitating complications, treatment for OCP involves systemic immunomodulation for active disease control as well as corneal and eyelid surgery for complications that fail to be controlled with topical or systemic medications.
When the acute phase of the disease is under control, scleral lenses and shell use may further benefit the ocular surface providing liquid bandage effect and mechanical protection. In a study conducted at Emory University, daily use of scleral lenses have been shown to improve keratopathy grades, prevent fornix shortening progression and improve vision in 20 patients (36 eyes) fitted for OCP management. The researchers found that 96% of fitted eyes responded well to therapy and continued scleral lens wear. They concluded that scleral lenses should be given a strong consideration as an ancillary therapy in patients with OCP.17
However, despite the underlying reason for the dryness, each of the prior-mentioned conditions can benefit from the presence of a scleral lens. Scleral lenses, when fit properly, do not maintain contact with the corneal surface, and rather keep a fluid reservoir between the lens and the cornea.17 This reservoir is filled with a nonpreserved saline solution prior to insertion, which serves to maintain sharp optical quality, avoid mechanical contact of the lens to the eye and additionally functions as a liquid bandage.6
Patients with severely dry and ocular surface inflammation can experience significant problems, from keratinization to corneal thinning and perforation.17,18 Often, a persistent ocular surface inflammation may lead to a formation of non-healing corneal epithelial defects, which in turn carry significant risk for infection with a consecutive damage to the cornea.18 Multiple studies have shown the efficacy of scleral contact lenses, as elucidated by the liquid bandage effect and mechanical protection from the eyelids, in successfully healing refractory corneal epithelial defects.17,19 Another potential benefit is the addition of a preservative-free antibiotic, such as moxifloxacin within the fluid reservoir of the scleral lens to serve as a prophylactic against further infection in these vulnerable eyes.20 Even in the setting of a cornea with significant dryness and no epithelial defect, the liquid bandage effect can help to promote corneal epithelial regeneration, reduce surface inflammation and redness and vastly improve patient comfort and vision.6,21 For any patients living with constant dry eye, scleral lenses can be considered as an adjunct therapy to improve their symptoms and ocular surface.
 |
|
Fig. 3. Scleral lenses in setting of OCP. Note the presence of trichiasis and symblepharon and conjunctival keratinization. Click image to enlarge. |
New Materials
The therapeutic benefits of the scleral lens greatly help improve patient quality of life; however, certain considerations regarding the lens should be taken. Firstly, with extended wear time of the lenses, one may consider the oxygen permeability (Dk) of the contact lens. With corneal gas permeable (GP) lenses, there is less of a concern regarding Dk, since the tear film is freely moving underneath the lens, allowing for oxygen exchange under the lens surface.23 However, with a scleral lens, there is almost no or limited tear exchange due to the fitting philosophy, particularly when the fitting aims to achieve better scleral alignment such as quadrant specific or profilometry-based scleral lenses.22 In such cases, although patient comfort with the lens may be improved as a result, fresh tear and oxygen ingression under a scleral lens is reduced. With this consideration in mind, there are newer hyper Dk GP lens materials available at our disposal.
Commonly used GP scleral lens materials include Boston XO and Boston XO2, with Dks of 100 and 141 correspondingly, that had proven results over the last 10 to 15 years.23 One of the newer GP materials being recently developed is Acuity 200, which boasts a hyper Dk of 200.24 Studies that investigated hypoxia-induced corneal swelling have found statistically significant reduced corneal edema on eyes wearing material with a Dk greater than 150 when compared to lower Dk materials, an important consideration for more vulnerable eyes requiring scleral lenses for vision, surface rehabilitation, such as after corneal transplant or with overnight wear.12 Although it may seem simple to continuously choose higher Dk materials, it is important to consider the potential non-wettability issues of a lens as the Dk increases, and the increased deposits that may occur as the oxygen permeability of the lens increases.2,24 Secondly, scleral lens replacement should be encouraged every one to two years, especially for more vulnerable eyes (post-penetrating keratoplasty, OCP, Stevens-Johnson syndrome, etc.), due to the decrease in oxygen permeability of GP materials.25
Finally, extended wear use of the scleral lens should only occur for therapeutic indications, such as ocular pain or persistent epithelial defect management. Overnight use will typically involve 12/12-hour wear of two separate lenses that are alternately used and cleaned.27 Altogether, it can only be advantageous to have a variety of materials to choose from in order to provide the healthiest balance for our patients, but proper wear and care need to be thoroughly discussed and understood by the patient.
 |
| Click table to enlarge. |
New Solutions
As the potential patient population that may benefit from scleral lenses increases, so do the tools at our disposal to use in conjunction with these lenses. Typically, the bowl of a scleral lens is filled with a preservative-free sterile sodium chloride solution prior to insertion. However, when we consider the natural tear film composition, there are a variety of minerals and electrolytes present in the liquid component (potassium, calcium, magnesium, phosphate and bicarbonate, etc). These solubles maintain the integrity of corneal epithelium and help keep the pH of the tear film balanced at about 7.0.11 A non-buffered solution such as sterile sodium chloride has an acidic pH closer to 5.5, which can lead to discomfort in some sensitive patients.9
New buffered additives to commercially available saline solutions, such as Nutrifill (Contamac) and Scleralfil (Bausch + Lomb), contain ions such as boric acid and magnesium while maintaining a pH of about 7.4 to mimic the natural tear film.21 When working with patients who have more sensitive eyes or a very low natural tear film, buffered solutions may be considered to improve wear time and patient comfort. Lubricating agents that may help promote wettability of the scleral lens once on the eye include drops containing hyaluronic acid, which serves to enhance corneal epithelial healing, restores morphology and function of conjunctival goblet cells and reduces ocular surface inflammation.28
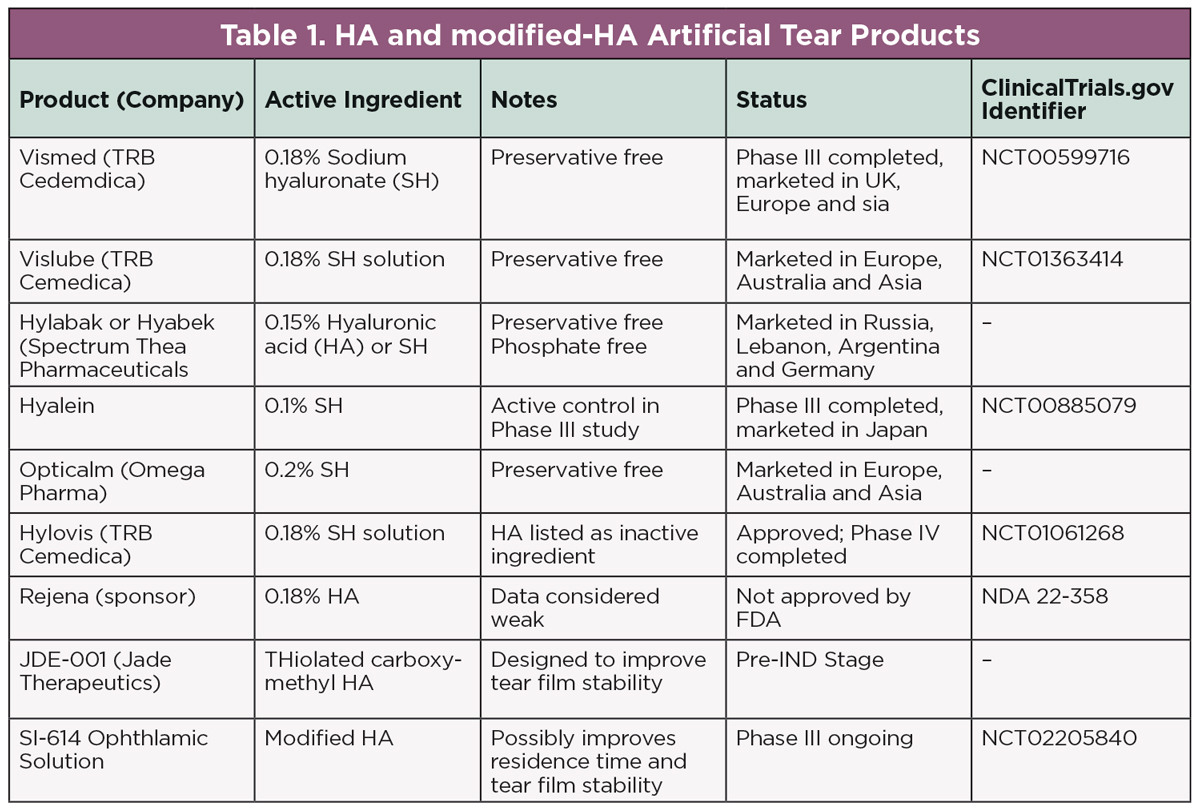
Preservative-free drops currently on the market containing povidone, hyaluronic acid or a derivative of it include Ivizia (Thea Pharma), Biotrue Hydration Boost (Bausch + Lomb), Optase Hylo and HyloGel (CandorVision). The surface tension created by hyaluronic acid allows the drop to function as a gel while the eye is open and as a less-viscous lubricant when blinking.28 This dynamic capability gives excellent functionality in dry eye patients who greatly benefit from reduced inflammation, improved ocular surface function and preservative-free formulation that allows for use with or without scleral lenses. An optimum hyaluronic acid concentration to achieve maximum tear film stability is recently being investigated in several clinical trials (Table 1).
The world of specialty lenses is truly exciting due to the forward-thinking technology constantly being proposed by its passionate researchers. As providers, we should think about applications of new technology to see if it can help us to better serve our patients. With the advent of customized aberration control, imaging technology made to expedite the fitting process, finer lens modifications, more breathable lens materials and a bounty of new wetting drops and solutions, there is no shortage of materials to experiment with.
Dr. Severinsky is an assistant professor at Emory School of Medicine and practices at Emory Eye Center in Atlanta, where he focuses on fitting specialty contact lenses. He is a fellow of the American Academy of Optometry, the British Contact Lens Association and the Scleral Lens Society.
Dr. Shafi has just completed her residency at Emory Eye Center and is now at University of California, San Francisco, where she is continuing her passion for anterior segment disease.
1. Nau CB, Schornack M. Scleral lens settling in 20 minute intervals over two hours with three lens designs. ARVO 2017 annual meeting. 2. Fogt JS, Schornack M, Nau C, et al. Image- and impression-based technology in scleral lens fitting for keratoconus: availability and utilization. Eye Contact Lens. 2024;50(7):292-6. 3. Yoon H, Harthan JS, Skoog W, et al. Process and outcomes of fitting corneoscleral profilometry-driven scleral lenses for patients with ocular surface disease. Eye Contact Lens. 2024;50(3):132-7. 4. Shafi A, Severinsky B. Predicting dual-elevation scleral lens rotation based on front corneal elevation map. Global Specialty Lens Symposium 2024. 5. Kojima R, Fujimoto M, Caroline P, et al. Sagittal depth measured on one subject by seven instruments. Global Specialty Lens Symposium 2024. 6. Kumar P, Carrasquillo KG, Chaudhary S, Basu S. A multi-parameter grading system for optimal fitting of scleral contact lenses. F1000Res. 2022;11:6. 7. Carracedo G, Serramito-Blanco M, Martin-Gil A, et al. Post-lens tear turbidity and visual quality after scleral lens wear. Clin Exp Optom. 2017;100(6):577-82. 8. Walker MK, Lema C, Redfern R. Potential pro-inflammatory impact of scleral lens midday fogging on human corneal epithelial cells: an in vitro study. Cont Lens Anterior Eye. May 17, 2024. [Epub ahead of print]. 9. Reddi BA. Why is saline so acidic (and does it really matter?). Int J Med Sci. 2013;10(6):747-50. 10. Nixon AD, Barr JT, VanNasdale DA. Corneal epithelial bullae after short-term wear of small diameter scleral lenses. Cont Lens Anterior Eye. 2017;40(2):116-26. 11. Fogt JS. Midday fogging of scleral contact lenses: current perspectives. Clin Optom. 2021;13:209-19. 12. Nau CB, Schornack MM, McLaren JW, et al. Tear exchange, intraocular pressure and wear characteristics of quadrant-specific vs. spherical haptic scleral lenses. Eye Contact Lens. 2022;48(11):460-5. 13. Hastings GD, Applegate RA, Nguyen LC, et al. Comparison of wavefront-guided and best conventional scleral lenses after habituation in eyes with corneal ectasia. Optom Vis Sci. 2019;96(4):238-47. 14. Guo M, Diaz GM, Yu Y, et al. Association between systemic medication use and severity of dry eye signs and symptoms in the dry eye assessment and management (DREAM) study. Ocul Surf. 2024;32:112-9. 15. Kam KW, Di Zazzo A, De Gregorio C, et al. A review on drug-induced dry eye disease. Indian J Ophthalmol. 2023;71(4):1263-9. 16. Shafi A, Severinsky B. Therapeutic applications of scleral contact lenses in ocular cicatricial pemphigoid. Global Specialty Lens Symposium 2024. 17. Kate A, Deshmukh R, Donthineni PR, et al. Management of corneal perforations in dry eye disease: preferred practice pattern guidelines. Indian J Ophthalmol. 2023;71(4):1373-81. 18. Kate A, Singh S, Das AV, Basu S. Dry eye disease and risk factors for corneal complications in chronic ocular graft-vs.-host disease. Indian J Ophthalmol. 2023;71(4):1538-44. 19. Vaidyanathan U, Hopping GC, Liu HY, et al. Persistent corneal epithelial defects: a review article. Med Hypothesis Discov Innov Ophthalmol. 2019;8(3):163-76. 20. He X, Donaldson KE, Perez VL, Sotomayor P. Case series: overnight wear of scleral lens for persistent epithelial defects. Optom Vis Sci. 2018;95(1):70-5. 21. Ruiz-Lozano RE, Gomez-Elizondo DE, Colorado-Zavala MF, et al. Update on indications, complications and outcomes of scleral contact lenses. Med Hypothesis Discov Innov Ophthalmol. 2022;10(4):165-78. 22. Ichijima H, Cavanagh HD. How rigid gas-permeable lenses supply more oxygen to the cornea than silicone hydrogels: a new model. Eye Contact Lens. 2007;33(5):216-23. 23. Ompañ V, Oliveira C, Aguilella-Arzo M, et al. Oxygen diffusion and edema with modern scleral rigid gas permeable contact lenses. Invest Ophthalmol Vis Sci. 2014;55(10):6421-9. 24. Fisher D, Experience with Acuity Polymer’s Acuity 200 material. CL Spectrum. 2021; 36(10):44-5. 25. Tsai HY, Hsieh YC, Lin YH, et al. Fabrication of hydrophilic surface on rigid gas permeable contact lenses to enhance the wettability using ultraviolet laser system. Micromachines (Basel). 2019; 10(6):394. 26. Jones L, Woods CA, Efron N. Life expectancy of rigid gas permeable and high water content contact lenses. CLAO J. 1996 Oct;22(4):258-61.. 27. Asghari B, Carrasquillo KG, Kwok A, Sippel KC. Use of PROSE for long-term ocular surface support in patients with a permanent keratoprosthesis. Am J Ophthalmol Case Rep. 2023;32:101919. 28. Aragona P, Simmons PA, Wang H, Wang T. Physicochemical properties of hyaluronic acid-based lubricant eye drops. Transl Vis Sci Technol. 2019;8(6):2. |


