Bacterial keratitis is a serious ocular condition that can lead to vision loss if not treated promptly, appropriately and aggressively.1 According to the CDC, an estimated one million clinical visits occur annually in the United States due to keratitis.2 Significant risk factors include contact lens use and ocular surface disease. Contact lens risk factors can be further broken down into overnight lens wear, poor storage hygiene and infrequent storage case replacement.2,3
Treatment of bacterial keratitis begins with empirical management using frequent instillation of topical broad-spectrum antibiotics for coverage of both gram-positive and gram-negative pathogens. Common treatment starting points depend on the severity of the corneal ulcer and consist of monotherapy with fluoroquinolones or combination therapy with cephalosporins, aminoglycosides, vancomycin, amikacin or fortified antibiotics.1,4 This increased first-line usage of broad-spectrum antibiotics, however, has coincided with a significant rise in the number of bacterial pathogens resistant to antimicrobials.5
In this review, we discuss relevant trends in bacterial keratitis, including corneal pathogen prevalence, corneal pathogen susceptibility, antibiotic resistance and treatment strategy.
Prevalence
According to two long-term retrospective case reviews of microbial keratitis in the United States, gram-positive organisms were the most commonly isolated bacterial group, followed by gram-negative organisms.6,7 Both studies indicated Staphylococci as the most prevalent gram-positive organism and Pseudomonas species as the most frequently isolated gram-negative and overall organism.6,7 The individual proportions of cultured species differed slightly by study.6,7 Another study noted that methicillin-resistant Staphylococcus aureus (MRSA) comprised 20% of all isolates, while 5% of all cultured isolates were MRSA.6
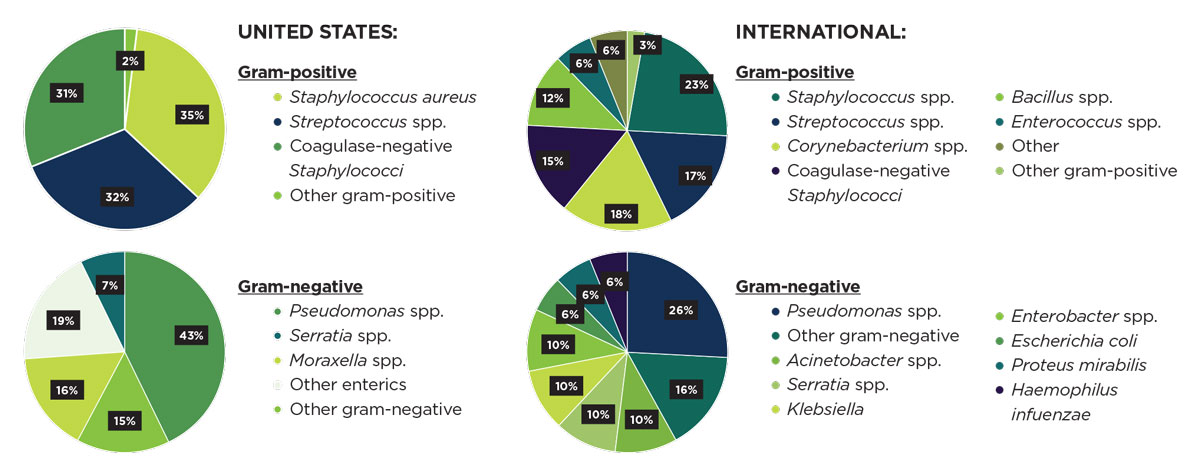 |
| Strain prevalence characteristics differ markedly between the United States and international regions (comprising India, China, Switzerland, Malaysia, South Korea, Taiwan, Spain, Colombia). Click image to enlarge. |
International studies show similarities and differences in bacterial prevalence compared with national findings.8-15 Similar to the United States, investigations in China, Switzerland, South Korea, Spain and Colombia indicate an overall preponderance of gram-positive organisms, as well as a predominance of Staphylococci and Pseudomonas individual strains.10,12,14,15 India, Taiwan and the tropics of Malaysia share another similarity with the United States—Pseudomonas was the single most commonly isolated bacterial organism.8,11,13 Of notable contrast to the aforementioned countries is Taiwan. From 2007 to 2016, Taiwan noted gram-negative bacteria as the most commonly isolated strain, followed by gram-positive bacteria.13
Temporal trends in the prevalence of gram-positive strains isolated from bacterial keratitis cases varied by each respective region. In the United States, there was a 1.13-increased odds of culturing MRSA for each one-year increase in culture date.6 The trends for gram-negative organisms also varied considerably by region and species. According to one study, the only bacterial pathogen that increased significantly in proportion during the study period was Pseudomonas aeruginosa.7
International trends in the proportions of bacterial isolates follow a similar route, differing by nation. There has been a significant increase in gram-positive isolates in India in recent years, in contrast with the decreasing trends in certain gram-positive strains observed in China.8,9 In India, there has been a decrease in the overall prevalence of gram-negative organisms, which differs from the increasing trends in certain gram-negative strains observed in China and Taiwan.8,9,13 Retrospective analyses conducted in Switzerland and South Korea indicate no difference in proportions of gram-positive and gram-negative organisms, as well as no discernible variation in causative pathogens responsible for microbial keratitis during their respective study periods.10,12
Susceptibility
Antibiotic sensitivity is a measure of the antibiotic concentration that will inhibit bacterial growth and is a predictor of the clinical response to antimicrobial treatment.16 Techniques of bacterial culturing, such as natural agar plates and corneal scraping, are used to determine the bacteria in question and its sensitivity.17 Knowledge of bacterial susceptibility can be helpful to guide the selection of antibiotics to achieve successful clinical outcomes.18
Retrospective analyses of gram-positive organisms conducted in the United States indicate significant variability in terms of antibiotic susceptibilities.6,7 Gram-positive isolates demonstrate 100% susceptibility to vancomycin over time and are fairly sensitive to gentamicin, tetracycline and trimethoprim sulfamethoxazole.6,7 However, susceptibility to fluoroquinolones, cefazolin and erythromycin is inconsistent.6,7 Furthermore, researchers note a decrease in susceptibility of gram-positive organisms to levofloxacin and gentamicin over time.6
Analyses of gram-negative organisms indicated excellent in vitro susceptibility of Pseudomonas to fluoroquinolones and aminoglycosides.6,7 Fluoroquinolones are also effective in Serratia, Moraxella and other enteric organisms.6,7 Non-Moraxella gram-negative rods exhibit better in vitro sensitivity to ceftazidime than to moxifloxacin and tobramycin.6
Internationally, studies document differences in antibiotic sensitivities and trends between the various regions. Gram-positive isolates have a high susceptibility to vancomycin, fluoroquinolones (including levofloxacin, moxifloxacin and gatifloxacin), aminoglycosides (namely, erythromycin, gentamicin and tobramycin), chloramphenicol and impinemen.8,9,12-15 In China, however, there has been a decrease in susceptibility of gram-positive isolates to levofloxacin, cefazolin, ceftazidime and chloramphenicol over time.9 Staphylococcus species in particular have a high antibiotic sensitivity to vancomycin, teicoplanin and chloramphenicol and a low sensitivity to fluoroquinolones, such as ciprofloxacin.8,13 Susceptibility of Streptococcus pneumoniae is highest to cefazolin and lowest to ciprofloxacin, which is mirrored by an overall trend of higher susceptibility of gram-positive cocci to cephalosporins relative to fluoroquinolones.8,9
In regard to gram-negative organisms, most are highly sensitive to fluoroquinolones (including ciprofloxacin and levofloxacin), aminoglycosides (namely gentamicin, amikacin and tobramycin), cephalosporins (including ceftazidime and cefepime) and carbapenem.9,11-13 Pseudomonas species in particular demonstrate excellent in vitro sensitivity to fluoroquinolones, aminoglycosides and certain cephalosporins.8,11-13
Studies conducted in South Korea and Taiwan do not show significant changes in antibiotic sensitivity over time.12,13 In comparison, analyses in China indicate a decreasing trend of susceptibilities. In China, fluoroquinolones were reported to be the most susceptible antibiotic to gram-negative bacilli, with an increased susceptibility to ofloxacin observed over time.9
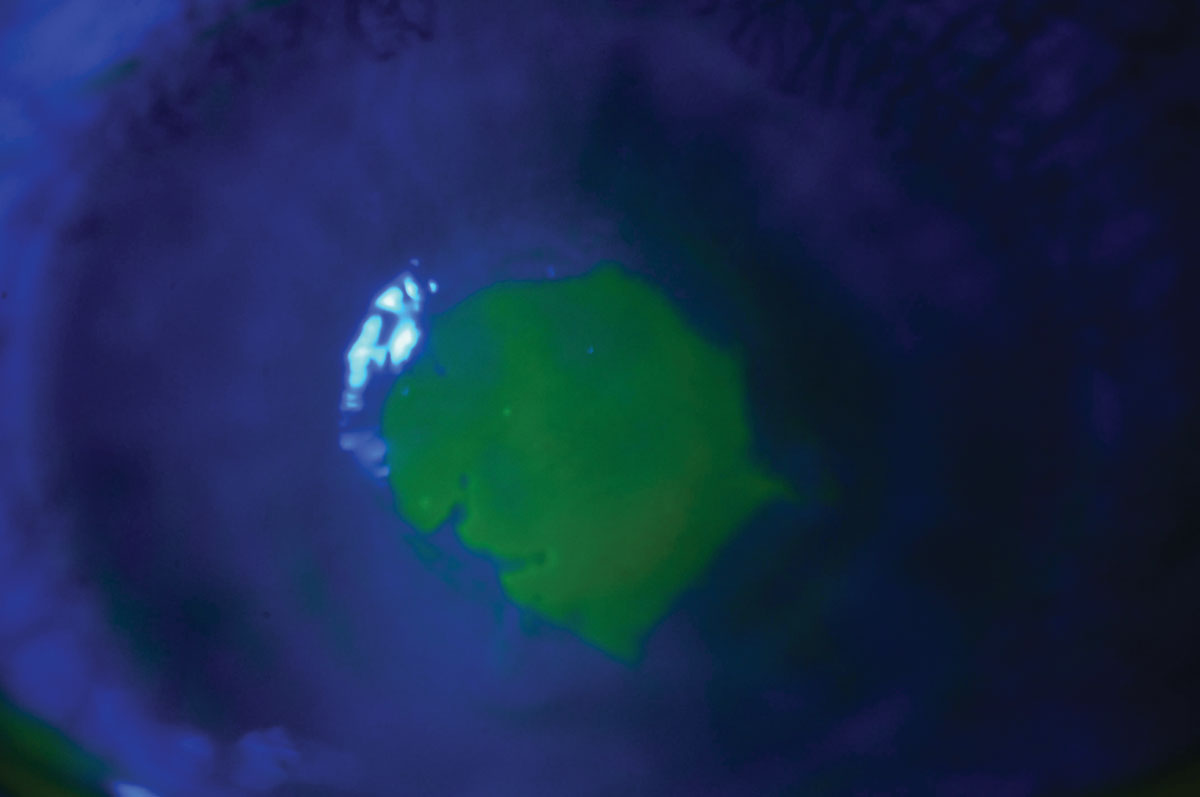 |
| Fluorescein staining shows an active bacterial ulcer in a patient who slept in her contact lenses. Click image to enlarge. |
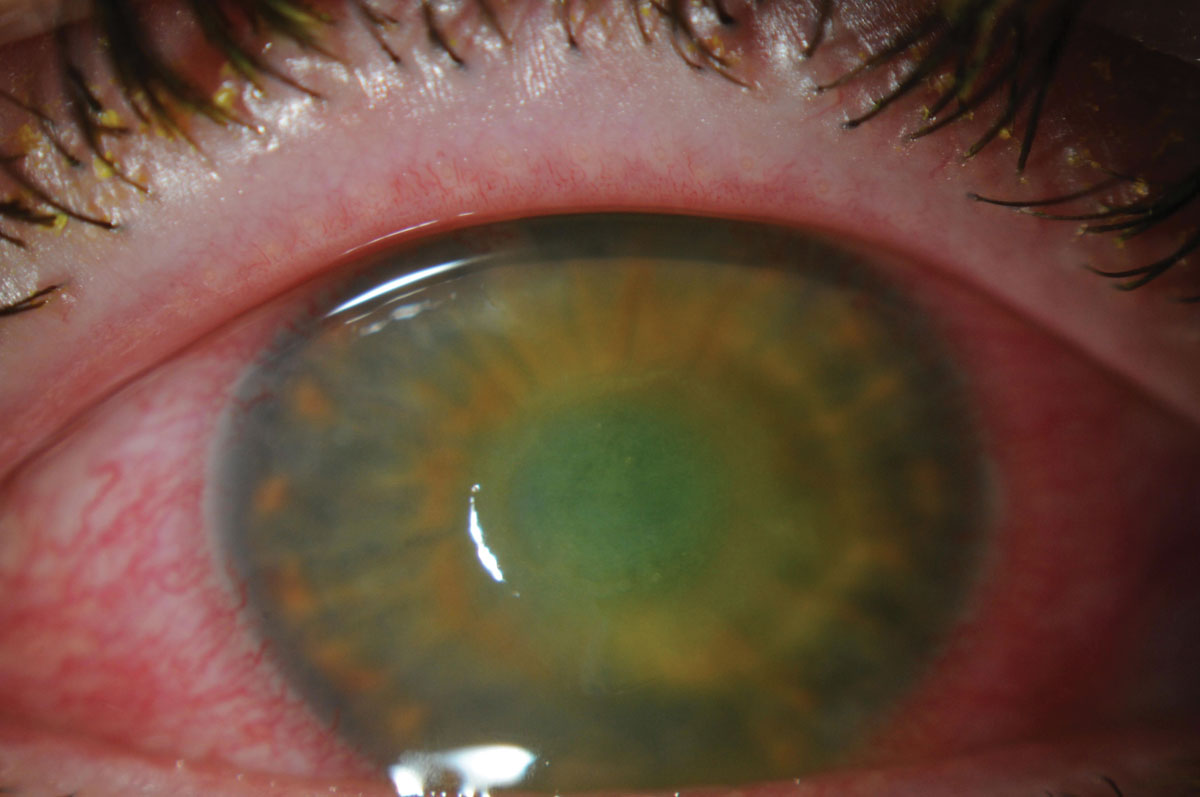 |
| The patient’s corneal ulcer is resolving. Click image to enlarge. |
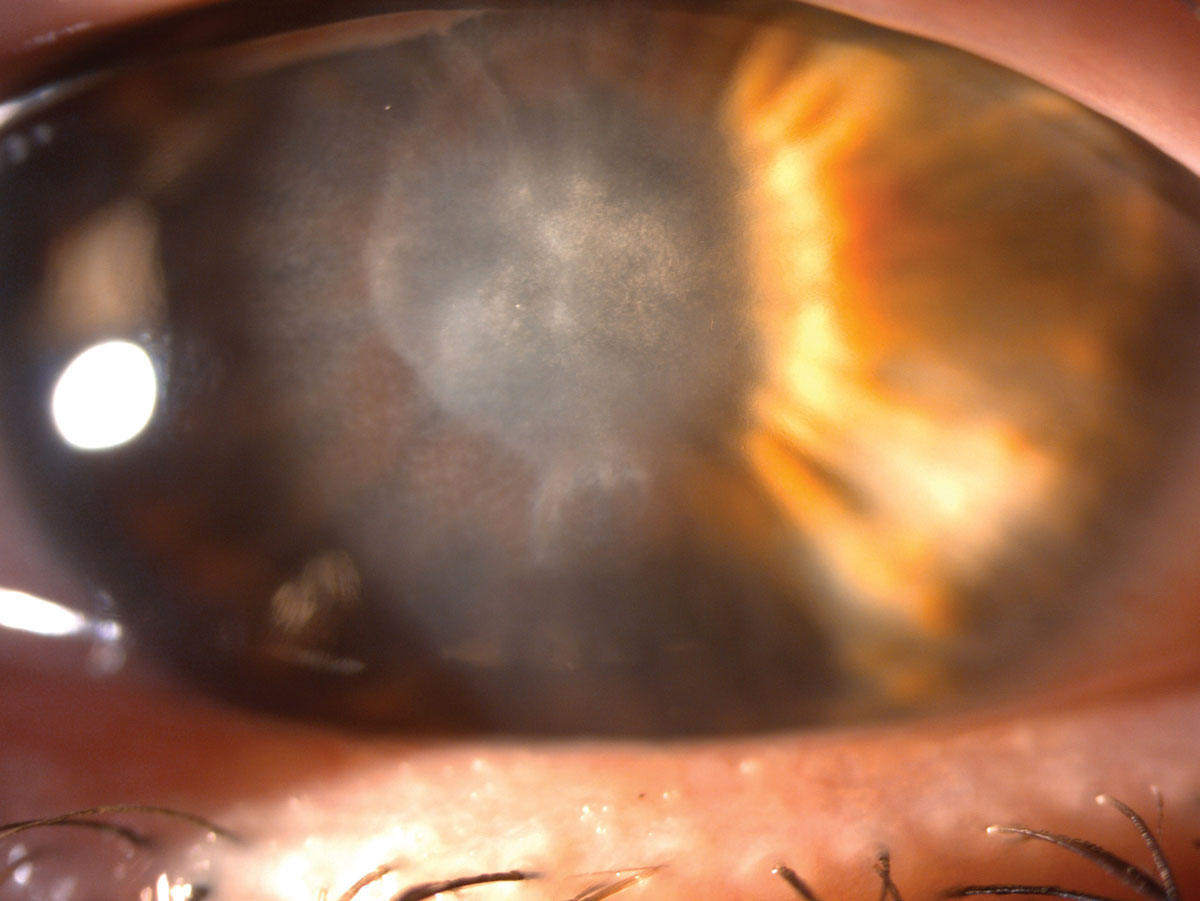 |
| She was left with this scar after her ulcer healed. Click image to enlarge. |
Antibiotic Resistance
This occurs when bacteria acquire the ability to evade destruction by antibiotics through spontaneous mutation or horizontal gene transfer. Resistance is driven largely by overuse of antibiotics. The resistance process removes drug-sensitive bacteria and leaves resistant strains to proliferate. Causes of antibiotic resistance include the vast number of antibiotics prescribed, incorrect prescribing of antibiotics, extensive agricultural use of antibiotics and the lack of availability of new antibiotics on the market.18 Trends in antibiotic resistance vary regionally, with certain bacterial organisms demonstrating increased resistance over time and other bacterial strains exhibiting no significant change in antibiotic resistance.
In the United States, trends in antibiotic resistance have changed over time and vary by region and bacterial strain. Resistance to moxifloxacin increases with each one-year increase in the culture date, and a trend of increasing resistance to gentamicin was observed among gram-positive organisms. Additionally, one study found the risk of culturing MRSA seems to increase with time in San Francisco, while no significant annual trends were noted in the proportions of oxacillin-resistant Staphylococcus aureus and oxacillin-resistant coagulase-negative Staphylococci in St. Louis.6,7
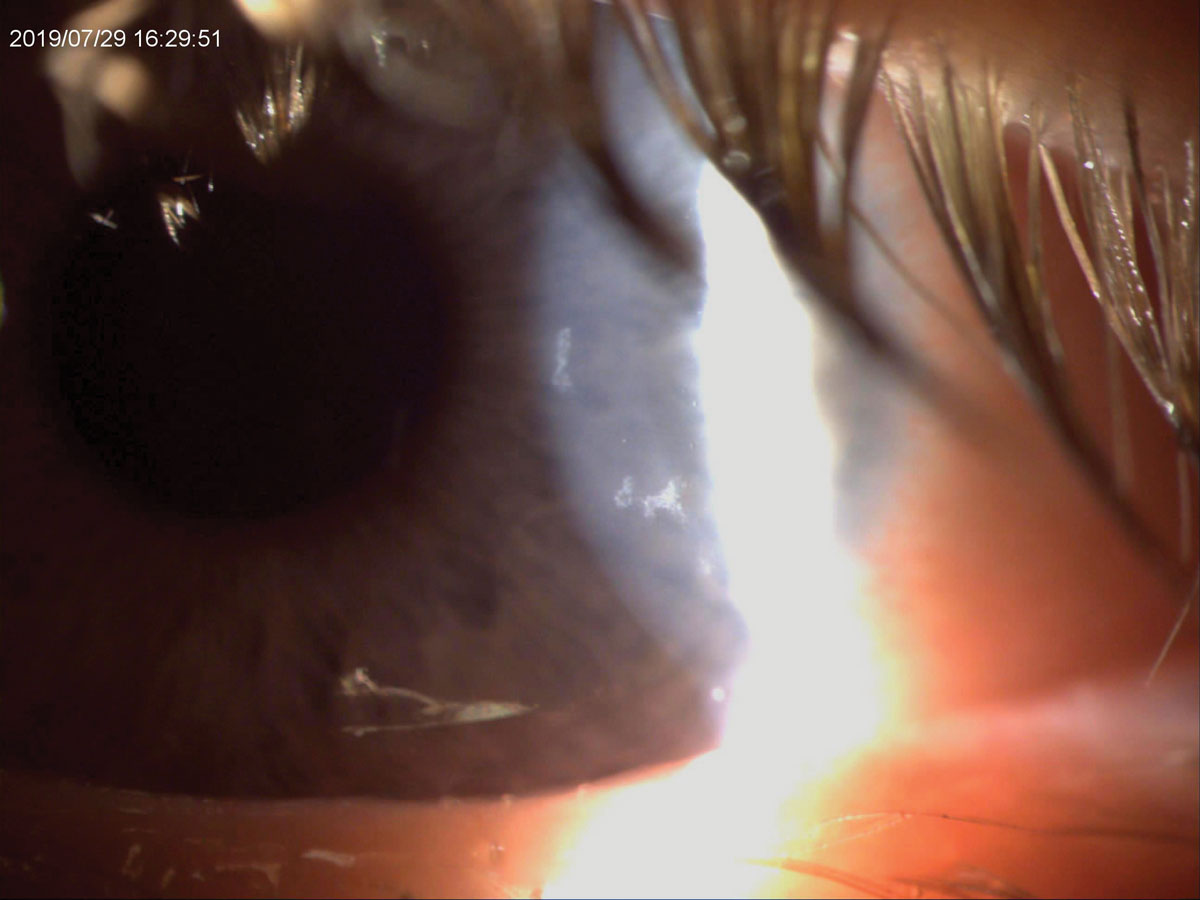 |
| This patient had irregular epitheliopathy at presentation and ended up having an active bacterial infection secondary to contact lenses. Click image to enlarge. |
Analyses in Malaysia observe no increase in resistance rates for the commonly used antibiotics, although these findings do not hold true for other regions.11 In Colombia, gram-negative organisms exhibit higher resistance rates overall, while in Taiwan, resistance is more common in gram-positive pathogens.13,15 Similar to the United States, the risk of culturing MRSA in Taiwan increases with time.13 This differs from findings in Spain and South Korea, where no trends have been observed for methicillin-resistant strains.12,14 Furthermore, Staphylococcus species demonstrate a significant increase in the proportion resistant to oxacillin over time. This is in contrast to the pattern seen in the United States.13 In Colombia and South Korea, moderate-to-high resistance is observed for gram-negative isolates to gentamicin, tobramycin, amikacin imipenem, gatifloxacin and ciprofloxacin, as well as for gram-positive isolates to Ciprofloxacin.12,15
Treatment Strategy
In addition to the current accepted methods used to treat microbial keratitis, a review of recent literature indicates several novel approaches may prove valuable for the treatment of bacterial corneal infections.
A broad-spectrum combination of polymyxin B–trimethoprim (PT) and rifampin demonstrated increased potency, improved antibiofilm activity and more rapid bactericidal activity compared with PT alone for the treatment of Staphylococcus aureus and Pseudomonas corneal infections.19 This novel combination also exhibits a lower tendency to develop resistance than either individual agent or moxifloxacin.19 Increased efficacy was also observed relative to commercial PT and moxifloxacin in murine models of keratitis.19
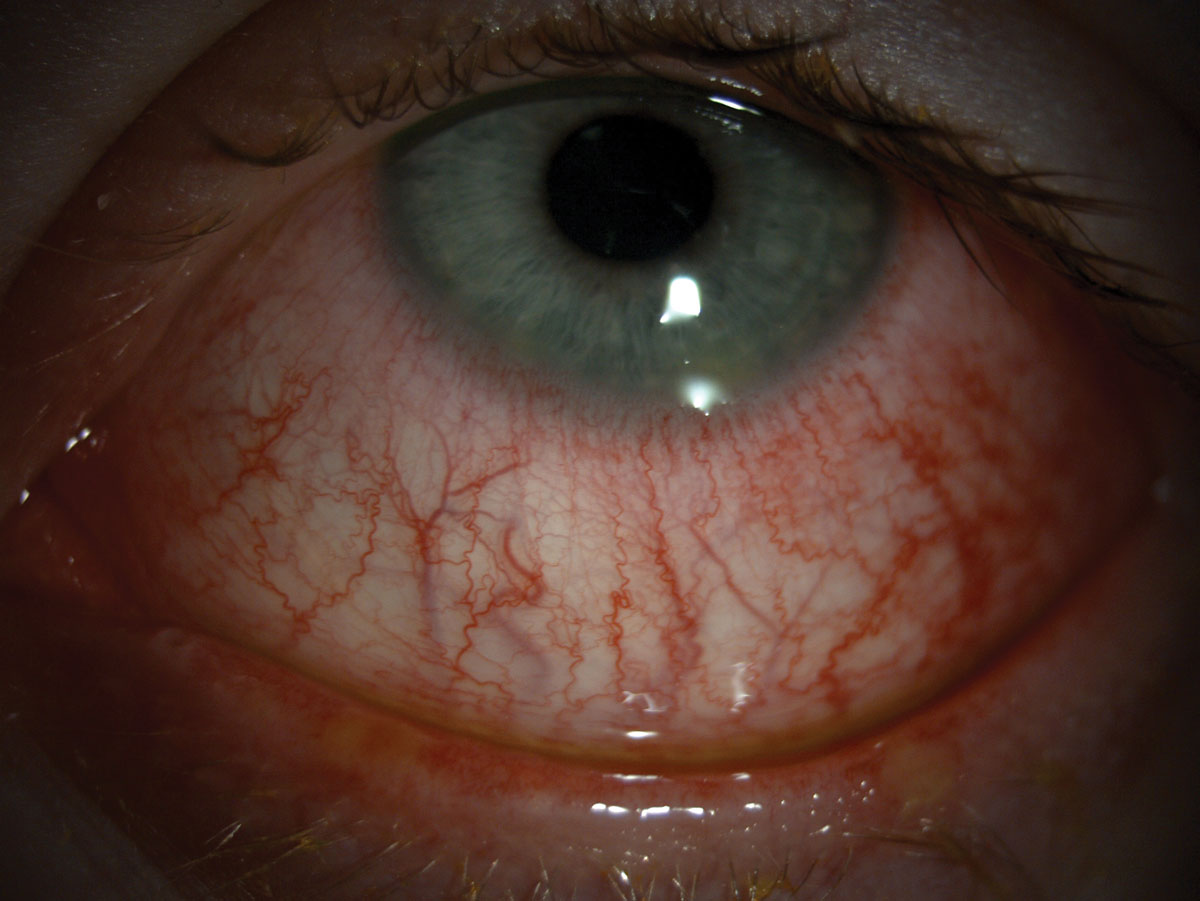 |
| Hyperemia can be seen in this patient. Click image to enlarge. |
Thymosin beta-4 (Tβ4) is a naturally occurring amino acid protein that promotes wound healing and host defense and reduces corneal inflammation. Corneas treated with a combination of Tβ4 and ciprofloxacin to fight off keratitis caused by Pseudomonas aeruginosa demonstrate the most improvement in disease severity, with minimal impact on host immune response.20
Researchers investigated argon cold plasma as a potential treatment for therapy-resistant corneal infections, specifically for Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli and Pseudomonas aeruginosa. Analyses indicate that argon cold plasma treatments can significantly reduce various corneal pathogens and improve vision post-treatment without damaging the ocular surface.21
In another study, patients with culture-positive bacterial keratitis—negative for fungal and protozoal species—received six or more daily drops of potent topical steroids, including prednisolone acetate 1%, phenylephrine hydrochloride 0.12%, dexamethasone 0.1% and prednisolone sodium phosphate 0.5%.22 Patients on the high-dose steroid regimen have a 5.5-increased chance of better visual outcomes, although patients with Nocardia keratitis had poorer outcomes.22 Treatment of bacterial keratitis with high-dose steroid regimens may prove to be an important clinical tool for improved visual outcomes after corneal infection.22
A working knowledge and understanding of common pathogens and their respective clinical therapies and current trends is essential to effectively manage bacterial keratitis and improve visual outcomes in patients. Given the widespread use of broad-spectrum antimicrobials and the subsequent emergence of antibiotic resistance, targeting various pathogens with treatments that have proven effective and using novel therapies adjunctively or in isolation may help improve success rates in the treatment of corneal ulcers and better preserve vision in those affected.
Ms. Cherny is a third-year optometry candidate at the SUNY College of Optometry, where she is completing an Advanced Cornea and Contact Lens micro-credential. She received her undergraduate Human Biology, Health and Society degree at Cornell University. She is interested in specialty contact lenses and corneal disease management.
Dr. Patel is a graduate of the SUNY College of Optometry. He completed a contact lens and ocular disease residency at The Ohio State University Havener Eye Institute. He is currently a clinical instructor at Weill Cornell Medicine Ophthalmology in New York City, where his focus is anterior segment management and specialty contact lens fitting. He is a fellow of the American Academy of Optometry.
Dr. Sherman is an assistant professor of optometric sciences in ophthalmology, director of optometric services at the Columbia University Medical Center and an assistant attending at New York–Presbyterian Hospital. She specializes in complex and medically necessary contact lens fittings, anterior segment disease and primary care. She is board-certified by the American Board of Optometry and National Board of Examiners in Optometry. She is a fellow of the American Academy of Optometry, conducts research, contributes to peer-reviewed scientific publications and presents at annual meetings.
1. Bourcier T, Thomas F, Borderie V, et al. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003;87(7):834-8. 2. Collier SA, Gronostaj MP, MacGurn AK, et al. Estimated burden of keratitis—United States, 2010. Morb Mortal Wkly Rep. 2014;63(45):1027-30. 3. Truong DT, Bui MT, Cavanagh HD. Epidemiology and outcome of microbial keratitis: private university versus urban public hospital care. Eye Cont Lens. 2018;44(Suppl 1):S82-6. 4. Acharya M, Farooqui JH, Jain S, et al. Pearls and paradigms in infective keratitis. Rom J Ophthalmol. 2019;63(2):119-27. 5. Odonkor ST, Addo KK. Bacteria resistance to antibiotics: recent trends and challenges. Int J Biol Med Res. 2011;2(4):1204-10. 6. Peng MY, Cevallos V, McLeod SD, et al. Bacterial keratitis: isolated organisms and antibiotic resistance patterns in San Francisco. Cornea. 2018;37(1):84-7. 7. Hsu HY, Ernst B, Schmidt EJ, et al. Laboratory results, epidemiologic features, and outcome analyses of microbial keratitis: a 15-year review from St. Louis. Am J Ophthalmol. 2019;198:54-62. 8. Das S, Samantaray R, Mallick A, et al. Types of organisms and in-vitro susceptibility of bacterial isolates from patients with microbial keratitis: a trend analysis of 8 years. Ind J Ophthalmol. 2019;67(1):49-53. 9. Lin L, Duan F, Yang Y, et al. Nine-year analysis of isolated pathogens and antibiotic susceptibilities of microbial keratitis from a large referral eye center in southern China. Infection and Drug Resistance. 2019;12:1295-302. 10. Bograd A, Seiler T, Droz S, et al. Bacterial and fungal keratitis: a retrospective analysis at a university hospital in Switzerland. Klin Monbl Augenheilkd. 2019;236(4):358-65. 11. Khor HG, Cho I, Lee KRCK, et al. Spectrum of microbial keratitis encountered in the tropics. Eye Cont Lens. 2019:1542-2321. 12. Mun Y, Kim MK, Oh JY. Ten-year analysis of microbiological profile and antibiotic sensitivity for bacterial keratitis in Korea. PLoS ONE. 2019;14(3):e0213103. 13. Liu HY, Chu HS, Wang IJ, et al. Microbial keratitis in Taiwan: a 20-year update. Am J Ophthalmol. 2019;205:74-81. 14. Tena D, Rodríguez N, Toribio L, et al. Infectious keratitis: microbiological review of 297 cases. Jpn J Infect Dis. 2019;72(2):121-3. 15. Galvis V, Parra MM, Tello A, et al. Antibiotic resistance profile in eye infections in a reference centre in Floridablanca, Colombia. Arch Soc Esp Oftalmol. 2019;94(1):4-11. 16. Smaill F. Antibiotic susceptibility and resistance testing: an overview. Can J Gastroenterol. 2000;14(10):871-5. 17. Leck A. Taking a corneal scrape and making a diagnosis. Comm Eye Health. 2009;22(71):42-3. 18. Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. Pharm Therapeut. 2015;40(4):277-83. 19. Chojnacki M, Philbrick A, Wucher B, et al. Development of a broad-spectrum antimicrobial combination for the treatment of Staphylococcus aureus and Pseudomonas aeruginosa corneal infections. Antimicrob Agents Chemother. 2018;63(1):e01929-18. 20. Carion TW, Ebrahim AS, Kracht D, et al. Thymosin Beta-4 and ciprofloxacin adjunctive therapy improves Pseudomonas aeruginosa-induced keratitis. Cells. 2018;7(10):145. 21. Reitberger HH, Czugala M, Chow C, et al. Argon cold plasma—a novel tool to treat therapy-resistant corneal infections. Am J Ophthalmol. 2018;190:150-63. 22. Green M, Hughes I, Hogden J, et al. High-dose steroid treatment of bacterial keratitis. Cornea. 2019;38(2):135-40. |


