Scleral lenses have always been part of the scene, especially when treating diseases of the ocular surface or restoring vision in patients with irregular corneas or ectasia. While they were once reserved for an elite of prescribers, technological development has made it possible to democratize their use over the past 10 years. Now, there are thousands of practitioners around the world who offer this modality, which is slowly but surely becoming a standard offering in a contact lens practice.
While it is true that, not long ago, fitting scleral lenses was more an art than science, multiple research and clinical studies made a difference. We have now a better understanding of the anatomy of the ocular surface and its relationship with scleral lenses. Using the devices at our disposal, it has never been easier to fit scleral lenses. Today, it’s even common to proceed with a totally empirical approach by deriving lens parameters from the profiles obtained via mapping devices, which are increasingly reliable. The lens design itself has been refined, now offering adjustment possibilities by quadrant and zones, all in order to minimize physiological impacts on the eye and optimize the vision provided.
All of these changes resolved a number of issues that existed in the past. They also generated other effects with which we have to learn to fix. Here, we discuss the most common problems and attempts to provide practical solutions to solve them.
Fitting Issues
It is now known that the sclera and the conjunctiva are asymmetric, toric, non-rotational surfaces.1 Almost three out of four eyes present a conjunctiva with irregular toricity (unrelated to the corneal form), which increases further away from the limbus.2 It’s not uncommon to see differences of 300µm to 500µm between ocular surface peaks and valleys. There is no such thing as two perpendicular principal meridians to describe conjunctival profile. Very rarely, the surface varies according to a predictable sinusoidal pattern. As a result, it is difficult to perfectly align the lens with the ocular surface unless a landing zone is derived from an optical or physical molding.3
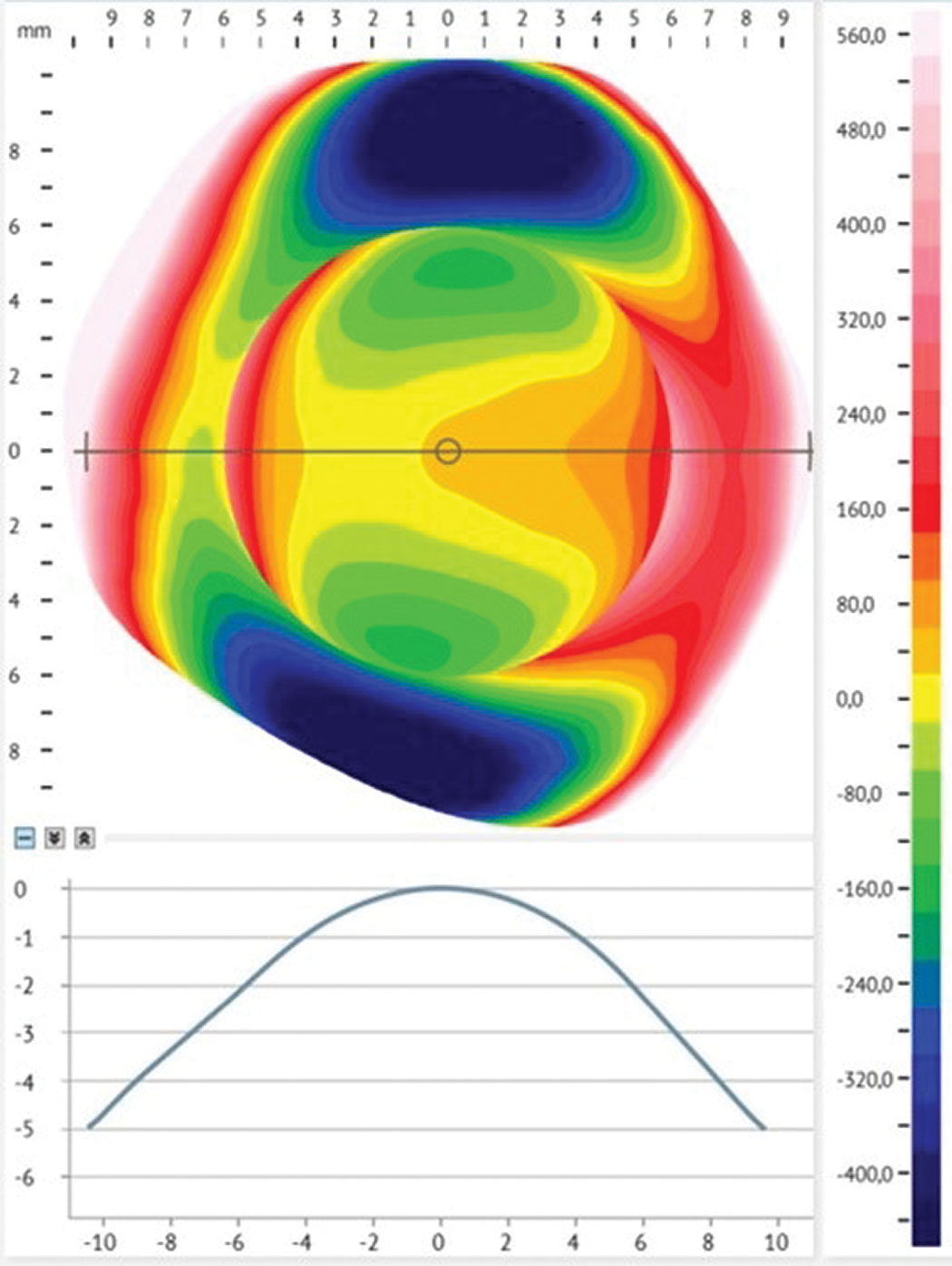 |
Fig 1. Profilometry of the conjunctiva shows irregular toricity of more than 450 um. Click image to enlarge. |
The issues depend on the misalignment that occurs in a given quadrant. When the lens is flatter than the surface, it lifts off, causing discomfort (lens-to-lid interaction). This open door also allows entry of debris in the reservoir, attracted by the sub-atmospheric pressure acting as a suction force. When substantial, debris contributes to mid-day fogging.4 One way to limit its presence is to better draw the peripheries of the lenses in order to obtain an impeccable alignment in all the meridians.
On the other hand, when the lens is too steep relative to the ocular surface, it generates compression of the tissue and blood vessels.5 Adjacent tissues can also become more swollen, increasing the compressive effect as hours of wear accumulate. The lens becomes difficult to remove at night, and rebound redness occurs within seconds of removal. Besides the aesthetic concern, this type of compression is associated with marked discomfort—a feeling of dry eyes and constant pressure felt in the eye.6 Usually, the patient cannot tolerate this type of long-term effect.
At the slit lamp, it is extremely difficult to assess the adjustments, in microns, that must be made when the lens-to-conjunctiva alignment is deficient. Another issue arises when we qualify these defects in terms of steps. A “step 2” curvature for one lab will imply a difference of 60µm from standard, while for another manufacturer it will mean 100µm. Therefore, it is strongly recommended to qualify in numbers (microns) and not in steps (step 1-2-3, flat 1-2-3) the changes required, so that all speak the same language.7
For reference, conjunctival toricity becomes significant when it reaches 300µm or more. To match this, scleral lens toricity must be designed with equivalent toricity. Change in peripheral curve toricity must reach at least 100µm to become clinically significant. With less than that, the lens will behave like one designed with spherical peripheral curves.
The best approach is to perform a conjunctival scan—before wearing scleral lenses—and to establish a reliable profile. Three devices exist for this purpose.8 They not only provide a topography of the conjunctival surface, but their software can also suggest the parameters of the first lens to be fitted. Some also offer the option of forwarding the scans to the lens manufacturer where consultants can help interpret them and design an optimal lens for the patient.
Another advantage: by analyzing these scans, it is possible to predict lens rotation, and therefore its stabilization axis (usually the steeper). This makes it possible, in advance, to compensate for the orientation of the frontal toric power when the latter is required. It is therefore becoming increasingly easy and obvious that scleral lens fitting can and should be done empirically.9 As a result, practitioners gain efficiency and precision, while minimizing the chair time required for the multiple trials that the use of diagnostic lenses entails. This saved chair time amply justifies the investment in reliable instruments that allow the ocular surface to be measured with precision.
The use of conjunctival topographers also makes it possible to better define the limbus. The most recent research data indicates that the cornea is oval and that the elevation of the limbus is not symmetrical, like a potato chip.10 These differences generate variations in the thickness of the reservoir above the limbus. The vault over the limbus is a critical element in the presence of mid-day fogging and also of conjunctival prolapse.11 Therefore, it’s necessary to minimize the vault to limit the entry of debris and prevent the conjunctival folds from forming. Scans can indicate the need for a quadrant-specific limbal vault, which improves the overall fit of the lens.
Some of the manufacturers now offer the option of designing a toric, oval-shape optic zone, accommodating variations of the limbal elevation and diameter, as well as quadrant-specific peripheral curves that are independently designed.12 A better designed, better aligned lens will tend to be less off-center. However, the centration of the lens is a fundamental element in the visual correction of patients.
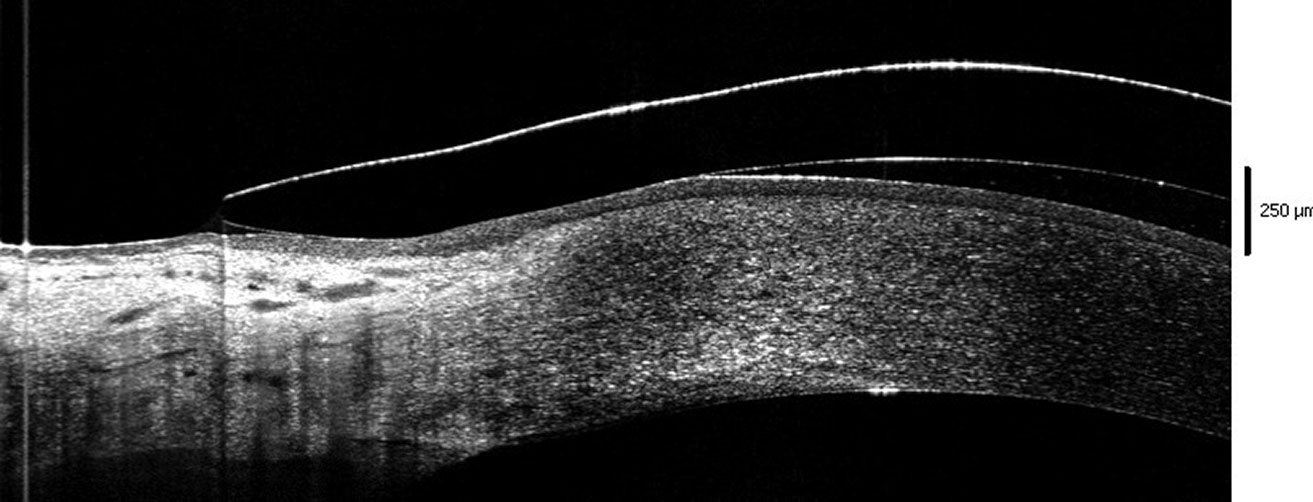 |
| Fig 2. Edge of the lens lifting off the conjunctival surface. Click image to enlarge. |
Visual Acuity Issues
One of the biggest frustrations I have when fitting scleral lenses is not being able to correct the patient’s visual acuity optimally. This is often the case with patients showing nipple cones or low/emerging oval cones, whose visual acuity in glasses is correctable to 20/30 or better. Fitted with sclerals, these patients complain of shadowing, ghosting of images and stretching of letters. Most of the time, they prefer their glasses to these new devices supposedly prescribed to improve their visual outcome.
Intuitively, these symptoms relate to the presence of residual astigmatism, coming either from the posterior cornea, crystalline lens or generated by a presumed flexure of the scleral lens.
Clinical studies have shown that it is quite different. Although a common recommendation from consultants, using a front toric lens will not help to improve visual acuity of these patients. Some practitioners may find signs of astigmatism when performing retinoscopy, but subjectively, if vision is slightly improved with toric over-refraction, there is never a “wow” moment associated with it. Increasing lens thickness to fix flexure does not provide any good outcome as it was also proven. Thin lenses fitted with low vault have the same behavior as thick lenses fitted with greater fluid reservoir, and the same symptoms are found.13
Why is this? Simply because the true nature of the visual symptoms is neither physiological nor related to the lens parameters, but indeed manifestations of higher-order aberrations (HOAs), in particular vertical coma.14 They are highly disturbing because the patient never experienced them before, or most often HOAs naturally present in their eyes are of the opposite direction and partly suppressed, with the patient being used to it. The new ones, generated by lens wear, are consequently more disturbing and obvious.
There are several reasons for the presence of these aberrations. First, the multiple indices change at each interface of the media (air, tears, anterior lens surface, posterior lens surface, fluid reservoir, front cornea, back cornea and to a lesser extent aqueous humor), influencing the light passing through the eye.
The second reason is linked with the lens centration. Most scleral lenses—in particular the larger ones—will tend to decenter down and temporal, following gravity and the natural slope/shape of the conjunctival surface. Consequently, the optical axis of the lens is no longer positioned in front of the visual axis, generating HOAs. The fluid reservoir is also modified, now shaped as a prism, and contributing to the degradation of the image perceived.
Finally, in keratoconus, posterior and anterior cornea tend to compensate for each other in aberrations generated. When a scleral lens is worn, most of the anterior corneal surface is compensated and HOAs from the back surface are now fully expressed.15
There are several ways to fix these issues. We identified adding a front toric power or increasing lens thickness as not effective. Another, more promising avenue is to generate an optical surface that will compensate HOAs in the system. Topo-guided wavefront optics can be (not so easily) generated; a few manufacturers are beginning to offer this option.
Stabilization of the lens is crucial here. Any rotation or movement will modify the level and pattern of HOAs, and their compensation on the lens will then be useless. Another possibility is to increase anterior surface asphericity without generating convex power. It works well, but the difficulty is to determine the right level of asphericity for a given eye. Again, many trial-and-error sessions may occur and can generate frustration on both patient and practitioner sides. The easiest method to limit HOAs’ influence on acuity is to make sure the lens is not decentered. This means to align the lens with the conjunctiva after proper mapping of its surface, as discussed previously.
It may be intuitive as well to rely on a smaller diameter lens, which would be fit with a lower vault. For instance, a 15mm lens would—in theory—deal with less conjunctival toricity than a 17.5mm one; this is not true. The important factor to consider here is the primary functional diameter (PFD) of the lens rather than its overall diameter. PFD is determined by the first contact of the lens with the ocular surface. Manufacturers can determine this value and must disclose it.
It is not rare to see a 16mm lens landing at 14mm and a 15.5mm OAD landing at 13.5mm. Consequently, there is not much difference between these two lenses and both will behave about the same on the same ocular surface. The rest of the lens, over PFD, can be considered just plastic with very limited impact on the lens behavior and its support from the ocular surface, except if the lens is not designed with curves but with tangents (like PROSE or Visser’s design). For the latter, peripheral curves are more closely aligned and match the conjunctival profile whilst with curves, the lens is generating a series of touch point on the landing area, the first one supporting most of the lens mass. In the case of tangent designs, diameter matters, which is not the case for 90% of the other lenses in the market.
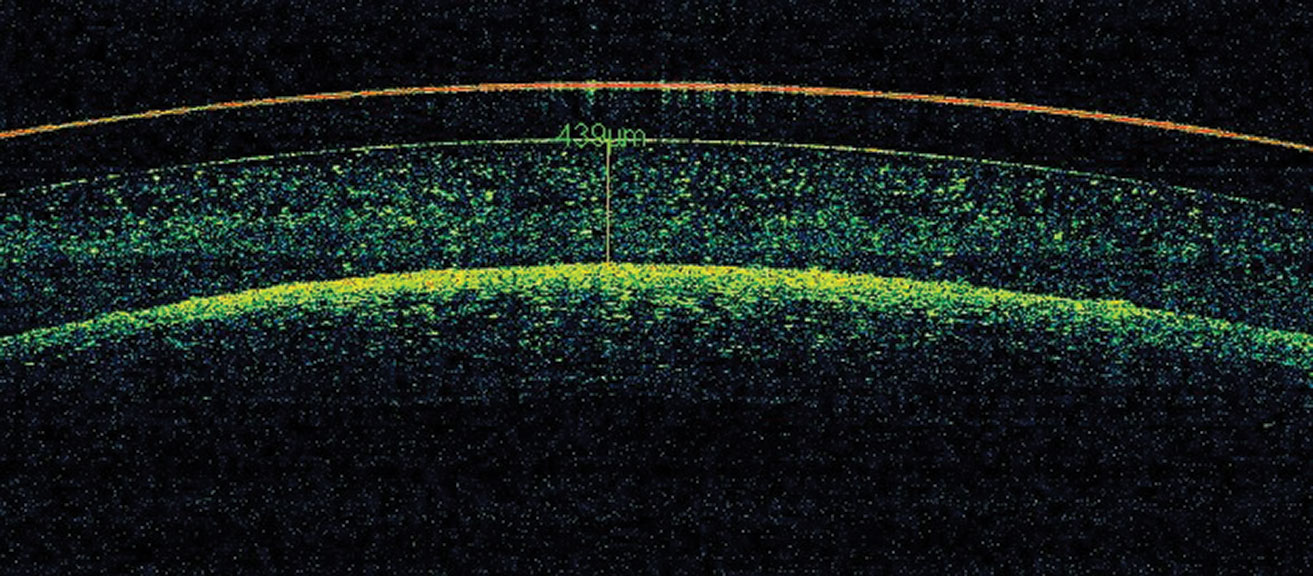 |
Fig 3. Debris is accumulated in the reservoir (mid-day fogging). Click image to enlarge. |
Physiological Issues
An article published in 2012 suggested a theoretical model to evaluate hypoxic stress related to scleral lens wear.16 Based on this, the cornea remains free of hypoxic stress if the lens was made thin (250µm or less) and fitted with lower vault (200µm or less), assuming that it was made with the highest Dk. This was a simple model looking at direct diffusion of the oxygen through the lens and the fluid reservoir, assuming that there was no tear exchange, no tear mixing and no lateral diffusion of oxygen in the cornea.
The article generated an ongoing debate and has since been cited more than 100 times, which says a lot about the importance of this topic and the extensive research that was carried out in the last 10 years.
We now know that the model is more complicated that was suggested. Oxygen diffusion is more complex and we have to take in account the carbon dioxide exchange as well, and the impact of both on the corneal metabolism.17 Limbal vessels may play a role that was not considered in the previous model. Consequently, the model must be revisited.
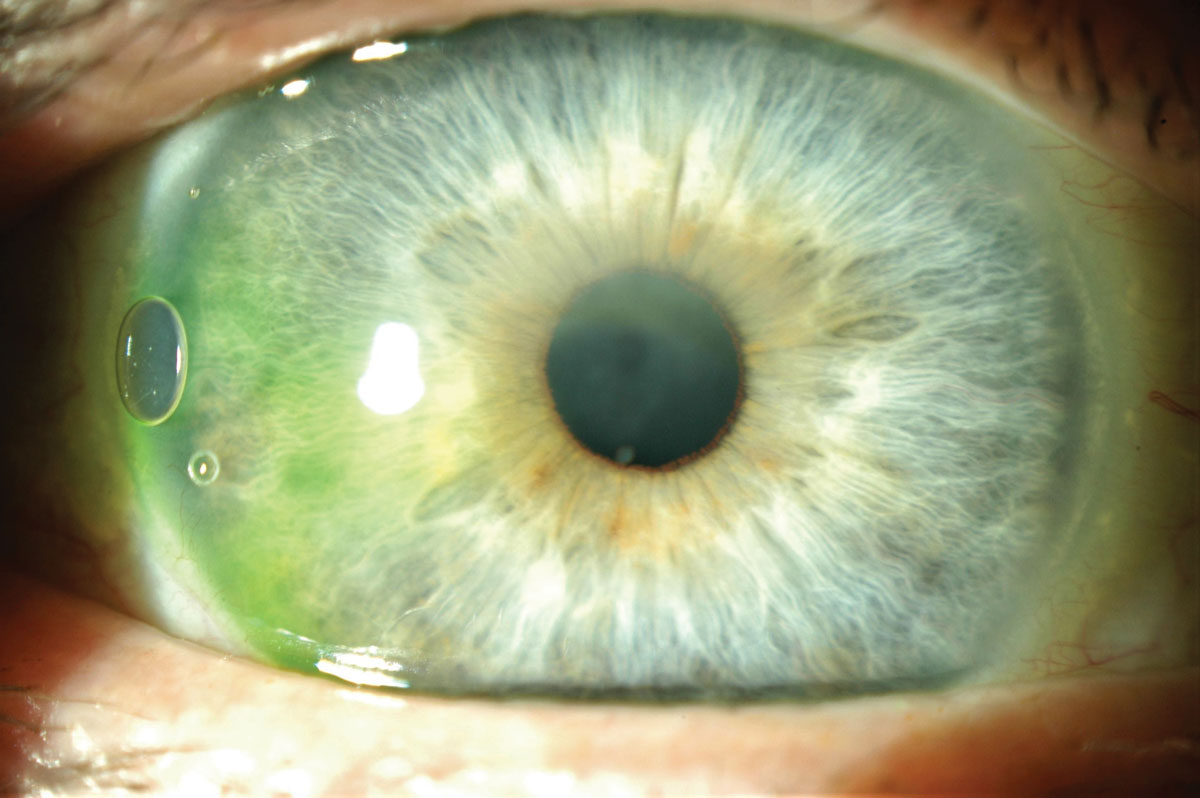
What became obvious is that corneal edema occurs with scleral lens wear very rapidly after lens insertion and stabilizes to reach a plateau after 90 minutes post insertion. Edema is stromal in nature, not epithelial, and is better evaluated through OCT, while the lens is worn compared to pachymetry once the lens is removed. The level of corneal edema never exceeds 4%, which is considered physiological, except if the lens is worn under closed-eye conditions.
On average, edema reaches 1% to 2%, which is considered safe for a normal cornea by most authors. This is probably where the debate begins. The first mistake is that we compare apples and oranges. Physiological edema reaches 4% after night eye closure but fades away over one hour upon awakening. The cornea is then restored to its normal metabolism. When scleral lenses are worn, they are applied after a few minutes of eye opening in the morning. Cornea is still edematous and will remain in this status during all wearing hours. Chronic hypoxic stress occurs with no known long-term impact.
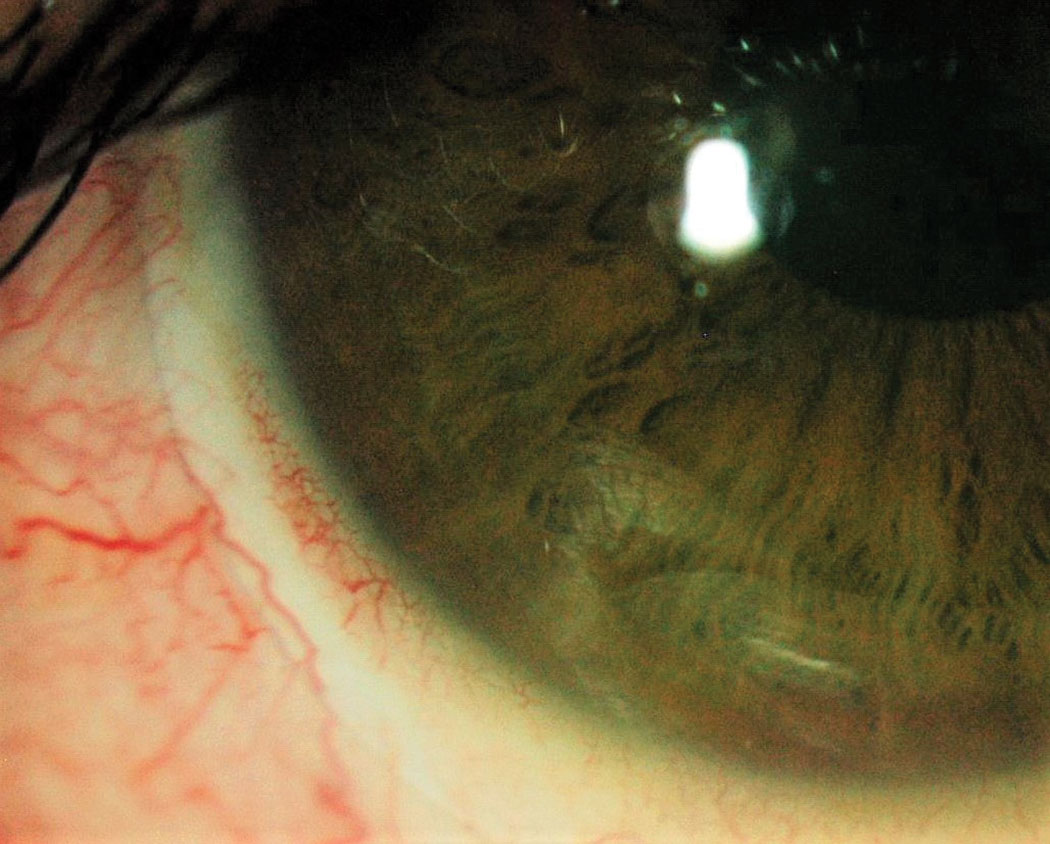 |
Fig 4. Conjunctival compression with blanching. Click image to enlarge. |
Oxygen permeability of a lens is evaluated by dividing the material permeability by its thickness. It is now possible to manufacture scleral lenses with very high Dk (up to 200), which must be favored. The lens thickness varies according to its design and its power. Minimal thickness is mandatory to alleviate lens break and to keep its geometrical stability. Optimal lens thickness varies from 200µm to 300µm.19 Another factor in play is the fluid reservoir. The same logic applies here: oxygen permeability of the reservoir is dictated by its thickness and the fluid permeability, which is very low (around 80).20
Studies showed that, between the two factors and up to 4% edema, lens material permeability is the most important one.17 Under chronic, severe hypoxia or when the fluid thickness is highly excessive (600µm to 800µm), the reservoir increases its impact on the corneal metabolism.20 However, at a very high vault (>800µm), there is a gradient between the temperature of the fluid at the corneal surface and the one lying just under the lens surface. This difference generates a tear mixing that does not exist otherwise, which can contribute a marginal increase in the level of oxygen delivered to the cornea.
Consequently, best practices these days strongly suggest prescribing scleral lenses with the highest DK/t possible, at least on fragile corneas. I am not comfortable leaving any other eyes under hypoxic stress, as minimal as it is, especially when you can fit better with zero hypoxic stress and still keep all the benefits of the scleral lenses.22
 |
|
Fig 5. Corneal edema is visible under the scleral lens. Click image to enlarge. |
The last element to discuss showed up on our radar a few years ago. Charles McMonnies suggested that, in theory, scleral lenses can induce a rise in intraocular pressure (IOP) by compressing episcleral veins and the subatmospheric pressure can cause lens tightness over time. Several clinical studies were carried out to validate this statement.23
Some of these studies tend to evaluate IOP during lens wear, either before complete lens settling (<4hrs), which may underestimate the real impact of the lens on the pressure, or with equipment that is highly variable (transpalpebral) or not practical (pneumotonometry).24,25 Others evaluated IOP just after lens removal, which led to normal findings.26 As soon as the pressure on the episcleral veins is removed, aqueous outflow is restored rapidly and IOP goes back to normal level in a few seconds.
Other studies looked at the influence of the lens diameter, assuming that a larger lens would compress the ocular structures.27 This is not true, and again, PFD of the lens is the key element to consider. A 16.5mm lens landing at 13.8mm will generate the same pressure on the episcleral veins as a 15.0 lens landing around the same area (13.5mm). Both lenses will contribute the same to vein compression. The suction effect will be the same if the reservoir vault is kept constant.
 |
|
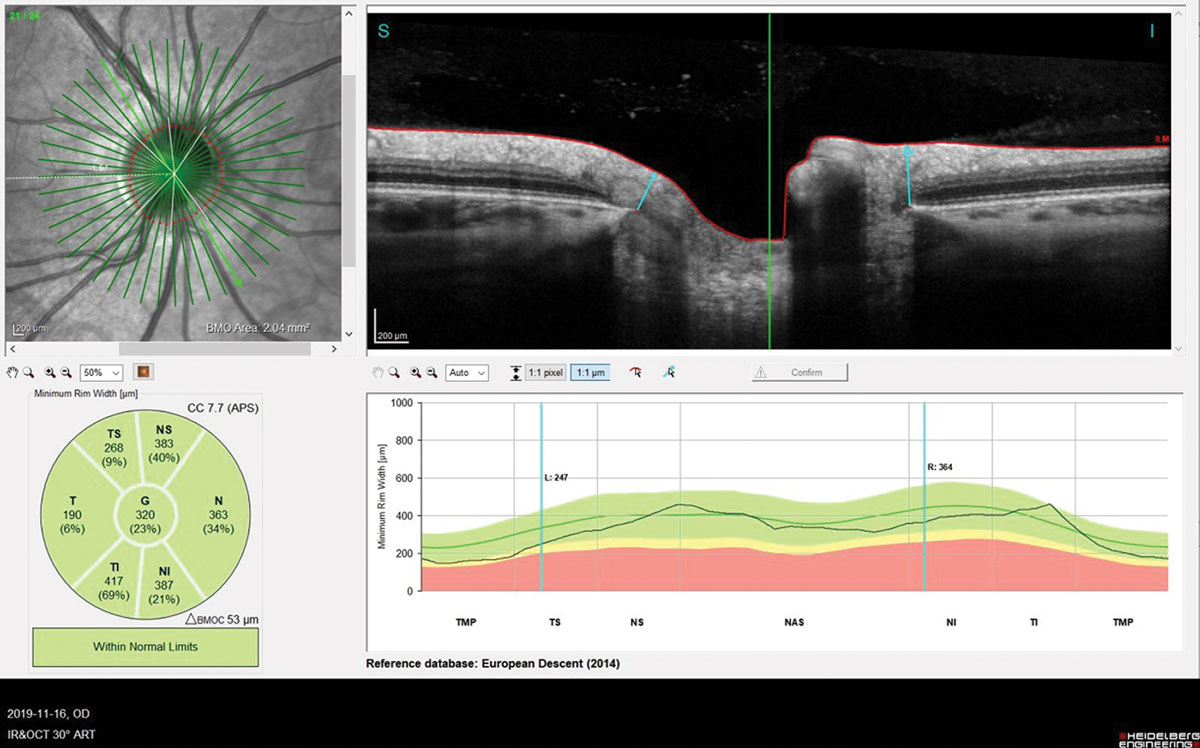
Fig 6. BMO-MRW assessed by OCT (Spectralis).Click image to enlarge. |
Finally, two manuscripts were published about the optic nerve reaction during lens wear, especially the Bruch’s membrane opening minimal rim width (BMO-MRW), which was found—at least in animal models—to be a valid marker of any IOP variation. One of these studies did find a slight variation of IOP (increase) but without reaching statistical significance.28 Just one eye was fit, the fellow one acting as a control, which may be not the state-of-the-art (synergistic effect). The other study took into account diurnal IOP variation and found a significant increase of IOP over six hours of lens wear (+5mm Hg).29 The same eye was compared to itself.
These conflicting results show the high variability among lens wearers. Nobody can predict who will spike in IOP and who will not be affected, but the reality is that some patients will indeed present a significant increase during scleral lens wear.
To fix this issue is quite simple: relieve some pressure by alleviating lens tightening over time. One thing is certain—scleral lenses would not generate glaucoma, at least on healthy optic nerve patients. For those already affected by glaucoma or at risk, caution is in order. In such cases, other options must be explored; if sclerals are finally selected, a close follow-up is mandatory, including optic nerve photo/scan analysis and visual field.
Takeaways
The last 10 years have given us incredible technological advances in terms of clinical data acquisition as well as the possibilities of manufacturing lenses. These innovations have multiplied the possibilities of adaptation to sclerals, which is beneficial for patients but can prove problematic for the practitioner, who must therefore juggle numerous options. It may be hard to master, especially for those fitting a limited number of cases per year. Some issues of the past persist, some have been solved and new ones are emerging. On each occasion, the practitioner must find ways to fix them. Hopefully, technology is there to help us.
Dr. Michaud graduated from L’École d’optométrie de Université de Montréal and is currently the dean of the School of Optometry. He is a Diplomate of the American Academy of Optometry and a Fellow of the British Contact Lens Association, the Scleral Lens Education Society and the European Academy of Optometry. He has received honoraria from Bausch + Lomb, CooperVision and Acculens.
1. Van der Worp, E., A Guide to Scleral Lens Fitting [monograph online]. Forest Grove, OR: Pacific University. 2010: Pacific University Libraries at CommonKnowledge. 63. 2. DeNaeyer G, Sanders D, van der Worp E, et al. Qualitative assessment of scleral shape patterns using a new wide field ocular surface elevation topographer. JCLRS. 2017; 1(1):12-22. 3. Nguyen MTB, Thakrar V, Chan CC. EyePrintPRO therapeutic scleral contact lens: indications and outcomes. Can J Ophthalmol. 2018; 53(1):66-70. 4. Skidmore KV, Walker MK, Marsack JD, et al. A measure of tear inflow in habitual scleral lens wearers with and without midday fogging. Cont Lens Anterior Eye. 2019. 42(1):36-42. 5. Consejo A, Behaegel J, Van Hoey M, et al. Scleral asymmetry as a potential predictor for scleral lens compression. Ophthalmic Physiol Opt. 2018; 38(6):609-16. 6. Walker MK, Bergmanson JP, Miller WL, et al. Complications and fitting challenges associated with scleral contact lenses: a review. Cont Lens Anterior Eye. 2016; 39(2):88-96. 7. Michaud L, Lipson M, Kramer E, et al. The official guide to scleral lens terminology. Cont Lens Anterior Eye. 2020; 43(6):529-34. 8. Macedo-de-Araujo RJ, van der Worp E, Gonzalez-Meijome JM. In vivo assessment of the anterior scleral contour assisted by automatic profilometry and changes in conjunctival shape after miniscleral contact lens fitting. J Optom. 2019; 12(2):131-40. 9. Fadel D, Barnett M, Arnold T, Sindt M. The future of scleral lens fitting. C.L. Spectrum, 2021. 36(April ): p. 38-43. 10. Sridhar MS. Anatomy of cornea and ocular surface. Indian J Ophthalmol. 2018; 66(2):190-4. 11. Mickles CV, Barnett M. Simple Tips for Troubleshooting Sclerals. Rev Cornea Cont Lens, 2016(April 15). 12. Fadel, D., Novel scleral lens design with elliptical and toric corneal and limbal zones, in GSLS 2018. 2018: Las Vegas 13. Michaud, L. and G. Courey, Influence of the scleral lens and fluid reservoir thicknesses on residual astigmatism. JCLRS [Internet], 2021(January ). 14. Hastings GD, Applegate RA, Nguyen LC, et al. Comparison of wavefront-guided and best conventional scleral lenses after habituation in eyes with corneal ectasia. Optom Vis Sci. 2019; 96(4):238-47. 15. Yoon G, Johns L, Tomashevskaya O, et al. Visual benefit of correcting higher order aberrations in keratoconus with customized scleral lens. Investigative Ophthalmology & Visual Science, 2010; 51(13):3432. 16. Michaud L, van der Worp E, Brazeau D, et al. Predicting estimates of oxygen transmissibility for scleral lenses. Cont Lens Anterior Eye. 2012; 35(6):266-71. 17. Kim YH, Tan B, Lin MC, et al. Central corneal edema with scleral-lens wear. Curr Eye Res. 2018; 43(11):1305-15. 18. Manning, J., Making sense of Scleral Lens Material. Rev Cornea Cont Lens, 2018(November 15). 19. Barnett, M. and G. De Naeyer, What is the appropriate amount of clearance?, in Scleral Lens Troubleshooting FAQs, E.S. Bennett, Editor. 2017: St Louis. p. 7. 20. Benjamin, W.J., Oxygen transport through contact lenses, in Contact Lens Practice, M. Guillon and M. Ruben, Editors. 1994, Chapmann Hall Medical Publishers: London UK. p. 47-69. 21. Fisher D, Collins MJ, Vincent SJ. Fluid reservoir thickness and corneal oedema during closed eye scleral lens wear. Cont Lens Anterior Eye. 2021; 44(1):102-7. 22. Kim YH, Lin MC, Radke CJ. Central-to-peripheral corneal edema during wear of embedded-component contact lenses. Cont Lens and Anterior Eye. 2021:101443. 23. McMonnies CW. A hypothesis that scleral contact lenses could elevate intraocular pressure. Clin Exp Optom. 2016; 99(6):594-6. 24. Nau CB, Schornack MM, McLaren JW, Sit AJ. Intraocular pressure after 2 hours of small-diameter scleral lens wear. Eye Contact Lens, 2016. 42(6):350-3. 25. Doherty MD, Carrim ZI, O’Neill DP. Diaton tonometry: an assessment of validity and preference against Goldmann tonometry. Clin Exp Ophthalmol. 2012; 40(4):e171-5. 26. Shahnazi KC, Isozaki VL, Chiu GB. Effect of scleral lens wear on central corneal thickness and intraocular pressure in patients with ocular surface disease. Eye Contact Lens. 2020; 46(6):341-7. 27. Michaud L, Samaha D, Giasson CJ. Intra-ocular pressure variation associated with the wear of scleral lenses of different diameters. Cont Lens Anterior Eye. 2019; 42(1):104-10. 28. Walker MK, Pardon LP, Redfern R, Patel N. IOP and optic nerve head morphology during scleral lens wear. Optom Vis Sci. 2020; 97(9):661-8. 29. Samaha D, Michaud L. Bruch membrane opening minimum rim width changes during scleral lens wear. Eye Contact Lens. 2021; 47(5):295-300. |


