 As primary eye care providers, we often face the challenge of patients presenting with chronic dry eye or anterior uveitis whose condition appears to initially improve, only to then inexplicably regress.
As primary eye care providers, we often face the challenge of patients presenting with chronic dry eye or anterior uveitis whose condition appears to initially improve, only to then inexplicably regress.
Despite our best efforts and the virtues of available medical therapy, we are unable to prevent this setback. Poor patient compliance due to cost issues and drug-related irritation could be a contributing factor to the variability of these conditions.
What if we could deliver an in-office, cost-effective and painless dose of concentrated medication, without the worry of potential negative side effects, such as increased intraocular pressure, keratitis or cataract formation? What if patients weren’t burdened by the need for frequent administration of eye drops and visits to the doctor because the medication that they needed could be delivered comfortably, quickly and efficaciously?
This scenario would be a clear win-win situation for both patients and providers alike. Well, iontophoresis may be the answer, and it could be a reality very soon.
How it Works
The concept of iontophoresis dates as far back as the early 1900s, but only recently have controlled clinical studies been undertaken to test the efficacy of the concept.1 Iontophoresis is a noninvasive process that allows a greater bioavailability of a given drug to reach the anterior and posterior segments than is normally possible with topical application.
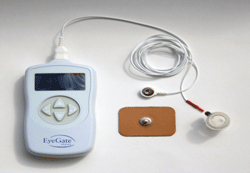
The EyeGate II generates a low-level electrical current that promotes the movement of a charged drug across biological membranes.
The process is a safer option than systemic dosing, which exposes the patient to the risk of system-wide adverse effects, or invasive intravitreal injection, which increases infection risk.
Ocular drug delivery via iontophoresis works on the principle that like charges repel and opposite charges attract, to allow more effective penetration through ocular tissues.2
In rabbit eyes, ionotophoresis demonstrated the ability to deliver greater than a 100-fold increase of drug concentration in the aqueous humor than was achieved with topical or intravenous administration.3 Safety of ocular iontophoresis in humans was demonstrated in a 2003 study conducted by Theodore Parkinson and colleagues.4
The EyeGate II delivery system (EGDS, Eyegate Pharmaceuticals) is an iontophoresis method of drug delivery in which an electrical field, generated by a low-level electrical current, promotes the movement of a charged drug across biological membranes. This process enables the delivery of negatively or positively charged therapeutics through tissues to targeted areas.
The amount of the drug that enters the eye can be controlled in two ways: the size of the current, and the specific length of time necessary for the treatment to deliver therapeutic levels of the drug into the eye while minimizing systemic absorption.
The device is equipped with an applicator containing a foam reservoir that houses the drug, which is placed just outside the limbus. The applicator is connected to a programmable generator, which is connected to an electrode that is placed on the forehead. The generator creates an electric field inside the applicator, and an opposite charge on the electrode, resulting in the propulsion of the drug into the eye.
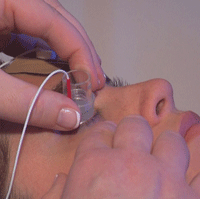
An applicator with a
reservoir that houses the drug is placed just outside the limbus. The
generator creates an electric field inside the applicator and an opposite charge on the forehead-based electrode, resulting in the propulsion of the drug into the eye.
EGP-437 is a novel 40-mg/mL dexamethasone phosphate specifically developed for use in iontophoresis for the treatment of dry eye and anterior uveitis.5 Topical steroids are typically used off-label to alleviate the signs and symptoms of dry eye,6 and are the standard of care for anterior uveitis.
Clinical Trials
EyeGate Pharma has recently completed two Phase III studies using the EGDS—one for dry eye and one for anterior uveitis:
• Dry eye: 198 patients were enrolled in a single center, nine-week, randomized, double-masked, placebo-controlled study, to evaluate the safety and efficacy of EGP-437. The researchers used the EGDS at two different dose levels: EGP-437 4.0 mA-min at 1.5 mA and EGP-437 6.5 mA-min at 2.5 mA, compared to iontophoresis with placebo (sodium citrate buffer solution).
The primary outcome measures were differences in corneal fluorescein staining at visit six compared to placebo and ocular discomfort at visit five, as compared to placebo. The improvement in inferior and total corneal staining were statistically significant at P=0.0084 and P=0.0196, respectively. EGP-437 also significantly improved symptoms (P=0.0058) at 24 hours post-treatment. The treatment was reported as both safe and well tolerated.7
• Anterior uveitis: In Phase II of this study, 40 randomized patients received an iontophoresis treatment in one qualifying eye. Patients were randomly placed into one of four iontophoresis dose groups (1.6, 4.8, 10.0 or 14.0 mA-min), treated with EGP-437 via the EGDS, and followed until day 28 of treatment. Two-thirds of the 40 patients enrolled achieved an anterior chamber cell score of zero within 28 days, after receiving only one iontophoresis treatment. Intraocular pressure remained within normal limits and there were no changes in visual acuity, nor were there any signs of cataract formation.8
Phase III, which was recently completed, was a randomized, double-masked, positive-controlled inferiority study that enrolled 193 patients at 45 clinical sites to assess the effectiveness of EGP-437 compared to topically applied prednisolone acetate 1% eye drops. Patients were randomized into either treatment with iontophoresis with EGP-437 on days 0 and 7, or 14 days of daily treatment with prednisolone acetate 1% eye drops, followed by two weeks of standard taper. The primary efficacy endpoint is the number of patients with anterior chamber cell count of zero on Day 14. Results were equivalent between the two groups; only two iontophoretic treatments resulted in 32/96 (33.3%) response and 32/97 (32.9%) of the prednisolone acetate eyedrop patients with no adverse events reported.9,10
The Path Forward
Conventional treatment of chronic dry eye, as well as anterior uveitis, requires multiple doses of eye drops over an extended period of time, sometimes taking weeks to months. In some cases, treatment can take years.
This approach to treatment can be costly, inconvenient and could also carry potential side effects. Iontophoresis appears to be capable of at least matching the effectiveness of topical steroids for anterior uveitis with a just a couple of treatments, and may provide the opportunity to use steroids more regularly and efficiently for a number of our recalcitrant dry eye patients.
1.Myles ME, Neumann DM, Hill JM. Recent progress in ocular drug delivery for posterior segment disease: emphasis on transscleral iontophoresis. Adv Drug Deliv Rev. 2005 Dec 13;57(14):2063-79.
2.Eljarrat-Binstock E, Domb AJ. Iontophoresis: a non-invasive ocular drug delivery. J Control Release. 2006 Feb 21;110(3):479-89.
3.Gungor S, Delgardo-Charo MB, Ruiz-Perez B et al. Trans-scleral iontophoretic delivery of low molecular weight therapeutics. J Controlled Release 2010;147:225-231.
4.Parkinson TM, Ferguson E, Febbraro S, Bakhtyari A, King M, Mundasad M. Tolerance of ocular iontophoresis in healthy volunteers. J Ocul Pharmacol Ther. 2003 Apr;19(2):145-51.
5.www.eyegatepharma.com.
6.Huang AJW. Immunosuppressive therapy for ocular surface disorders. In: Dry Eye and Ocular Surface Disorders. Pflugfelder SC, Beuerman RW,Stern ME; editors, New York, NY. Marcel Dekker, Inc. 2004.
7.http://www.clinicaltrials.gov/ct2/show/NCT0069135.
8.AE, Assang C, Patane MA, From S, Korenfeld M. Evaluation of dexamethasone phosphate delivered by ocular iontophoresis for treating noninfectious anterior uveitis. Ophthalmology. 2011;119(1):66-73.
9.http://www.clinicaltrials.gov/ct2/show/NCT01505088.
10.http://www.eyegatepharma.com/pdf/news2013/EyegatePR_Uveitis_TopLineData_08apr_Final.pdf.


