Contact lens fitting has always played a valuable role in the visual rehabilitation following keratoplasty. Many types of lenses can be used in the management of patients who have undergone corneal transplantation (ranging from full-thickness keratoplasty to anterior and posterior lamellar keratoplasties), including custom soft lenses, corneal gas permeable (GP) lenses, hybrid lenses, piggyback lenses and sclerals. However, these patients often present with varying degrees of corneal elevation, as well as high amounts of astigmatism and lack of symmetry, making the fit a challenge. As a result, many patients experience suboptimal vision and contact lens intolerance when fit with traditional soft or GP contact lens options. In these cases, scleral lenses in particular may be able to help.
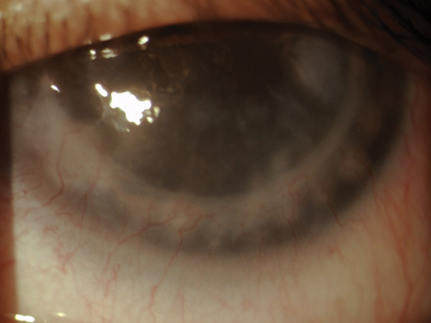 |
| This patient has stromal and endothelial graft rejection. Note the stromal edema and haze. The corneal neovascularization extends into two quadrants and was responsible for the rejection. |
Keys to a Post-op Fit
Keratoplasty procedures continue to evolve. Only the diseased or affected layers of the cornea are replaced during anterior and posterior lamellar procedures (deep anterior lamellar keratoplasty [DALK], Descemet’s stripping automated endothelial keratoplasty [DSAEK] and Descemet’s membrane endothelial keratoplasty [DMEK]) compared with the entire cornea during a full-thickness penetrating keratoplasty (PKP).
Complications have decreased and visual outcomes have improved with newer lamellar procedures.1-4 Patients who have undergone posterior lamellar procedures tend to have less surface irregularities compared with those who have had an anterior lamellar or PKP. Following a DSAEK or DMEK procedure, spectacles or traditional contact lens options tend to provide satisfactory visual improvement unless corneal scarring has occurred.5
Following a PKP, the epithelium is more fragile, and the endothelial cell density (ECD) is lower. Post-PKP corneas in particular are more prone to inflammatory events, increased risk of neovascularization and rejection.4,6
Contact lenses may be required following keratoplasty to enhance vision and to rehabilitate the ocular surface.7 These factors should influence the practitioner’s decision in selecting a contact lens.
Additional factors practitioners should consider when determining which type of contact lens to fit post-procedure include the amount of astigmatism present, any ocular surface disease, diameter, location and shape (prolate or oblate) of the graft, elevation between the host and donor cornea, and amount of corneal eccentricity.10-12 A contact lens fitting may also be needed to improve a patient’s anisometropia or aniseikonia.7
| The Procedures: PKP. During this procedure, the entire cornea is replaced with a donor cornea. PKP may be indicated for advanced keratoconus, bullous keratopathy, corneal dystrophies and degenerations, significant corneal scarring, contact lens intolerance or when all other means of correction have been exhausted.27-31 Post-procedure, patients are on chronic topical steroids and have an accelerated rate of endothelial decompensation. They are often dependent on specialty contact lenses due to irregular astigmatism and have a long visual recovery period.31 DALK. Here, all corneal tissue is removed except the recipient’s endothelium, Descemet’s membrane and, at times, deep stroma.27 Indications include keratoconus, corneal scarring and any corneal dystrophy or disease not involving the endothelium. Advantages over PKP include no risk of endothelial rejection, less residual astigmatism, faster visual recovery, increased graft survival and less long-term dependence on topical corticosteroids.32,33 DSAEK or DSEK. This involves removal of diseased endothelium, Descemet’s membrane and posterior stroma. Indications include Fuch’s dystrophy, pseudophakic bullous keratopathy, failed corneal graft and iridocorneal endothelial syndrome. DMEK. The newest form of endothelial transplantation, DMEK replaces one layer of endothelial cells and Descemet’s membrane (15μm to 20μm).27 Patients with uncomplicated cases of pseudophakic bullous keratopathy, Fuch’s and endothelial decompensation are potential candidates. Compared with PKP and DSEK, DMEK improves visual acuity and quality, as well as a lower rejection rate.1,34 |
While some patients have sutures remaining following keratoplasty, it is generally safe to proceed with contact lenses. However, protruding or exposed sutures may cause irritation, infection or stimulate neovascularization, so they must be removed promptly.1,7,13
Postoperative astigmatism is the main reason for unsatisfactory vision after keratoplasty. Studies indicate between 27% and 34% of grafts have more than 3D of corneal astigmatism two years after the procedure.10,14,15 Corneal topography or tomography should be performed on each post-keratoplasty patient to help determine which lens option is most suitable for the corneal shape.
The donor corneal graft is usually between 7.5mm and 8.5mm in diameter. Grafts that fall outside these parameters have lower survival rates and may complicate the contact lens fitting process.13 Grafts that are smaller than the pupil margin may cause vision distortion, and grafts close to the limbus have an increased risk of neovascularization and graft rejection.7
Many practitioners turn to GP contact lenses because they provide improved visual acuity, are able to correct for high amounts of astigmatism, have high oxygen permeability and are relatively low risk for the development of microbial keratitis and corneal neovascularization.11,15,16
Back surface or bi-toric GP lens designs have been used in some cases; however, due to the lack of rotational symmetry they are often not indicated as a first option. Instead, larger diameter corneal GP lenses (>10mm) are typically indicated so the back optic zone radius of the lens spans the graft-host junction.7 Reverse geometry designs are also common, especially in slightly protruding and oblate grafts, as they provide improved vision for patients with irregular corneal surfaces and elevation differences between the graft-host junction.12,16 These designs have one or more peripheral curves that are steeper than the base curve.7
The Case for Sclerals
Although corneal GP lenses are often the first choice for corneal transplants, they may not be the ideal option for certain complex cases. High variances in corneal curvature and elevation of the graft-host junction may cause GP lenses to decenter, leading to corneal staining and ocular inflammation.8
Corneal grafts that protrude slightly—as the graft is steeper than the host cornea—have a significant change in corneal curvature at the graft-host junction.7-9,14 When a practitioner fits these cases with corneal GP lenses, there may be edge lift and poor stability.7,14 This is where scleral contact lenses may be best suited.
| Density Matters ECD should be measured on all patients who have undergone keratoplasty prior to initiating the contact lens fitting. In a healthy adult patient, normal ECD is between 2,000cells/mm2 and 2,500cells/mm2.10 Corneas with ECD below 1000cells/mm2 are at an increased risk for swelling and decompensation.12 Chronic endothelial decompensation occurs when ECD is reduced to 400-700cells/mm2.12 For corneas that fall within this range, scleral lenses may be not be an option unless the benefits notably outweigh the risks. ECD decreases by approximately 30% following the procedure itself, and corneas that have undergone a keratoplasty decompensate at a faster rate than normal corneas.29,32,33 In patients who have undergone PKP, the average ECD is 800cells/mm2 at 15 years post-procedure, which is when the cornea starts to lose its clarity.2 When using a slit lamp, it can be difficult to measure small changes in the endothelium, especially during early stages of graft rejection or endothelial dystrophy. Practitioners should perform non-contact specular microscopy on patients who have had a corneal transplant at each follow-up visit because the instrument allows for quick and accurate visualization of endothelial cell counts.32,33 This can also be helpful in determining when to make contact lens fitting modifications for the patient or when to refer for further surgery.9 |
Scleral contact lenses are large diameter GP lenses (14mm to more than 20mm) that rest entirely on the sclera.15-17 Scleral lenses have become more regularly prescribed for the management of corneal irregularity, uncomplicated refractive error and ocular surface disease.10,18-21 In 2015, the Scleral Lenses in Current Ophthalmic Practice Evaluation study group reported that 16% of scleral lenses are being prescribed for ocular surface disease, 74% for corneal irregularity and 10% for uncomplicated refractive error.22
Scleral lenses may provide post-keratoplasty patients with all of the benefits of corneal GP lenses in addition to a well-centered, stable fit that vaults the cornea, protects the epithelial surface and improves comfort. Most custom labs manufacture scleral lenses, and impression prosthetic scleral devices designed to match the contours of the eye are now available for complex graft fits. Sclerals can be designed with spherical, front-surface toric, back-surface toric or bitoric optics to optimize both scleral alignment and visual acuity.10,23
While scleral lenses provide many benefits, corneal thickness and endothelial cell density (ECD) measurements should be performed on all keratoplasty patients prior to the contact lens fitting and during follow-up. For post-keratoplasty corneas that fall between 400cells/mm2 and 700cells/mm2, scleral lenses may be contraindicated unless the benefits outweigh the risks.
Scleral Design Choices
While scleral lenses can play an important role in healing or maintaining the ocular surface in post-transplant patients, practitioners need to keep a watchful eye. The use of scleral lenses for these patients is considered controversial due to decreased oxygen transmission and unknown long-term complications.24,25 Here, practitioners can use topographic or tomographic maps, such as elevation maps, to review the patient’s profile and identify whether or not the graft is more prolate or oblate prior to fitting.
Corneas with a prolate shape are steeper centrally and flatter in the periphery whereas oblate corneas are flatter centrally and steeper in the periphery.7,9,14 A corneal graft that has an oblate shape may require a reverse geometry or oblate scleral lens design.
Practitioners should use an optic section of the slit lamp or anterior segment optical coherence tomography to determine the amount of central corneal clearance during the scleral lens evaluation. Central corneal clearance can be estimated by comparing the center thickness of the lens with the thickness of the post-lens tear film reservoir with white light. If excessive central vault exists, practitioners should select a diagnostic lens with decreased sagittal depth. Conversely, if there is central touch, a higher sagittal depth may be needed.
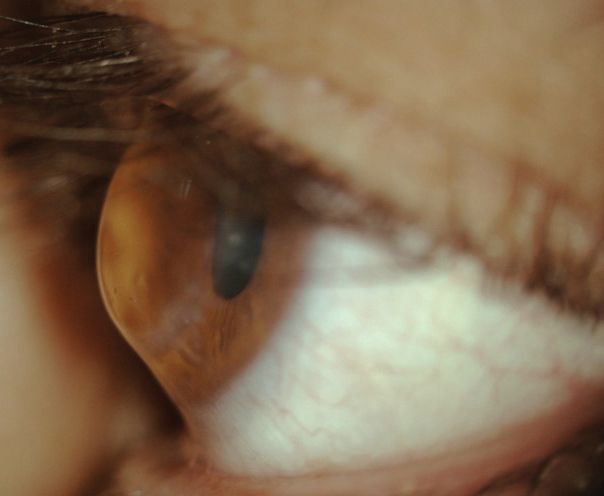 |
| The corneal profile of a post-PKP patient, demonstrating a prolate shape. |
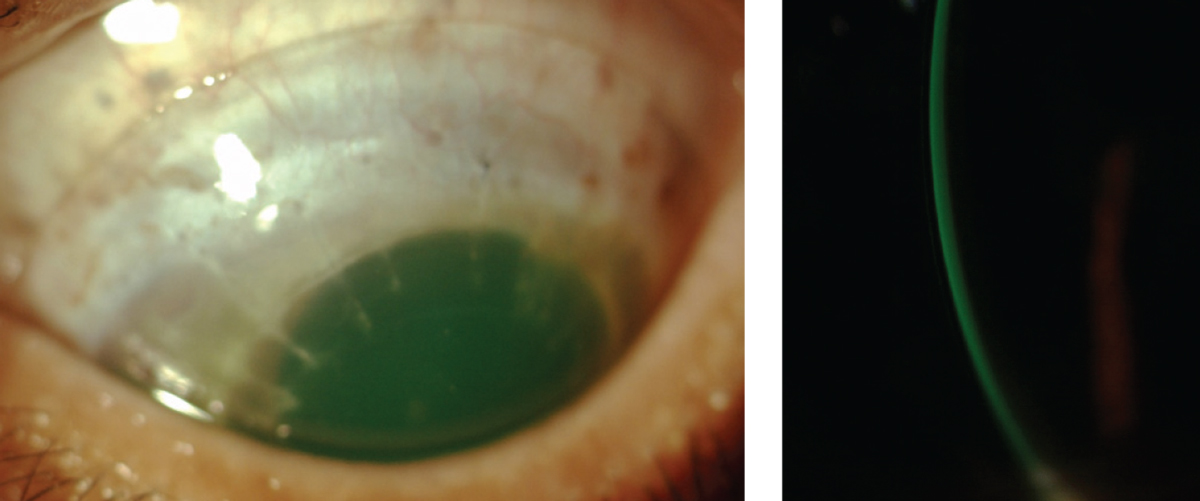 |
| Example of an acceptable scleral lens fit on a post-PKP patient. Central clearance is 200μm and there is no haptic blanching or compression. |
Scleral lenses are thicker than corneal GP lenses and a post-lens tear reservoir is created between the lens and the cornea, potentially contributing to physiological edema at a subclinical level.24-26 One study reported that the post-lens tear layer should be no greater than 200μm to avoid corneal edema using a high Dk (>150μm) lens with a maximum central thickness of 250μm.25
Once the central clearance is adequate, the next step is to ensure sufficient limbal clearance 360 degrees. Ideally, the lens edge or haptic should be aligned with the sclera. Excessive edge lift can be tightened while areas of vascular compression can be loosened. A toric peripheral system (some designs have four quadrant options available) may provide better alignment and centration.10,15 Once fit, the fluid-filled lens protects the epithelium and masks irregular astigmatism, improving best-corrected visual acuity.
Post-fit Pearls
Post-keratoplasty patients fit with a scleral lens require close monitoring (every three to four months) for signs of corneal hypoxia, neovascularization or transplant rejection. In addition to careful slit lamp examination, baseline and follow-up corneal pachymetry and endothelial cell counts are essential. If signs of corneal swelling or hypoxia arise, modifying the scleral lens fit or prescribing a shorter wear time may help. If additional troubleshooting is needed, practitioners can flatten the scleral lens haptic to increase tear film exchange, use a higher Dk lens material (>150μm) or introduce fenestrations.
Scleral lenses are quite versatile, and clinicians should not shy away from their use with this patient population. With a firm grasp of the post-keratoplasty eye and the many contact lens parameters that can be adjusted to provide an optimal fit, clinicians can fit scleral lenses to provide visual and therapeutic enhancements for their complicated corneal transplant patients.
Dr. Harthan is an associate professor at the Illinois College of Optometry and chief of the Cornea Center for Clinical Excellence at the Illinois Eye Institute. She lectures, publishes and participates in research on OSD and contact lenses.
1. Melles GR. Posterior lamellar keratoplasty: DLEK to DSEK to DMEK. Cornea. 2006;25:879-81. |


