Imagine if all homes were built with the same cookie-cutter design and material no matter the location, elevation or environment. Would every home last? Certainly not. Some materials and designs can work well for most, but every homeowner and plot of land has different needs. The same is true for scleral lenses, whose advancements and popularity have made some of us believe that any design can provide a solution to unique ocular problems. However, the more fittings you complete, the more unique challenges you will encounter, whether related to patient management or lens troubleshooting. Most patients will be satisfied with the vision scleral lenses can provide, but redness, fogging and inadequate comfort can lead to multiple adjustments and remakes. One design does not fit all, and we should look more carefully beyond the limbus to find solutions.
Most scleral lenses are designed with a common foundation (Figure 1). Successful fitting starts with understanding how to modify the multiple peripheral curves and base curve when challenges arise. Here’s guidance on designing these curves to avoid potential trouble from a poor fit.
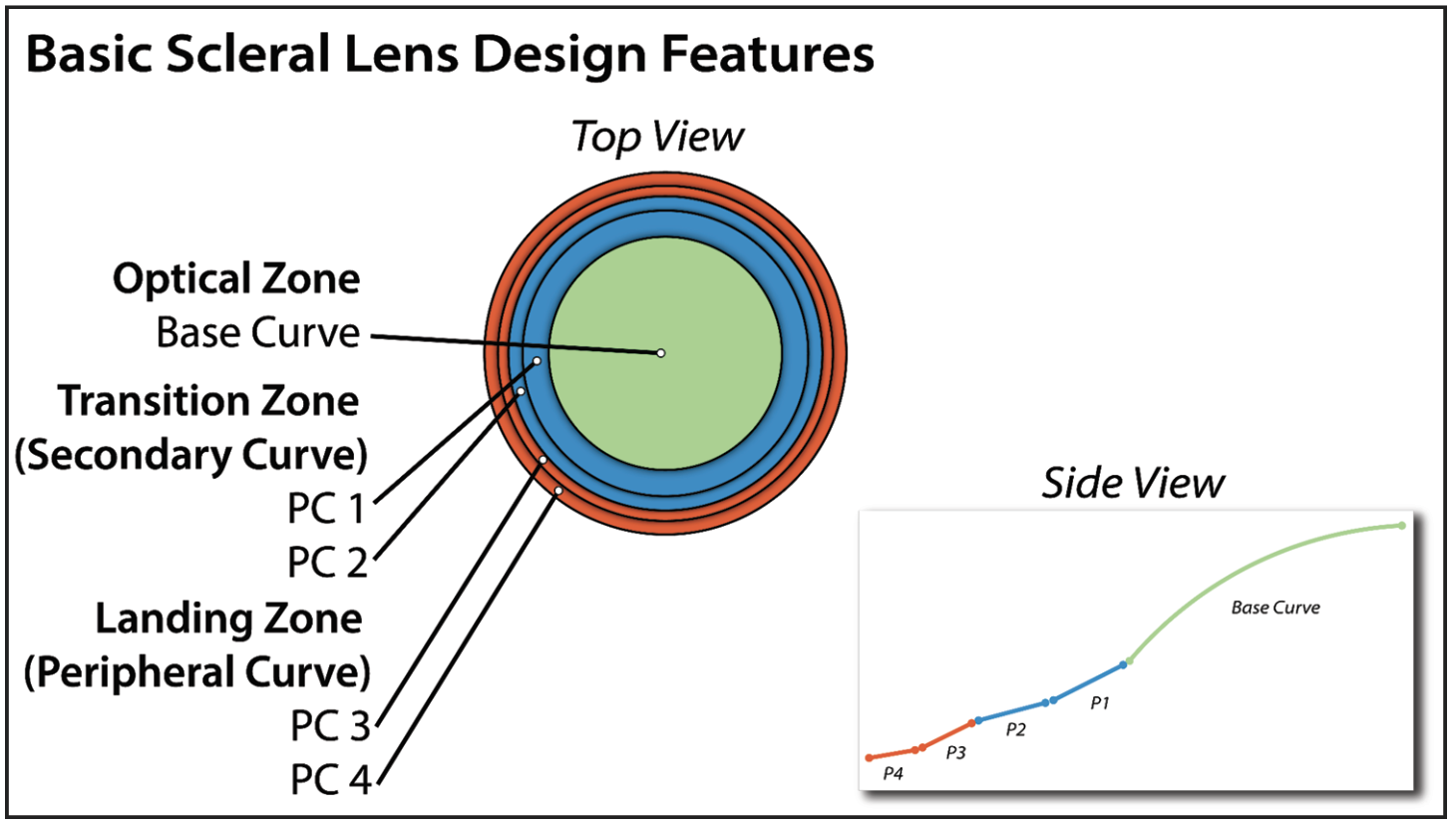 |
|
Fig. 1. Most scleral lenses share these common design features. Click image to enlarge. |
Scleral Shape Variability
A key principle to remember is that the further beyond the limbus you go, the more asymmetric the sclera becomes. Evidence of this comes from the Pacific Scleral Lens Study, which looked at multiple eyes of normal and keratoconic patients to assess the differences in scleral shape.1 Two chord lengths—measured as the total diameter between one edge and the other, similar to horizontal visible iris diameter—were used to assess scleral shapes; namely, 15.0mm and 20.0mm (Figure 2).
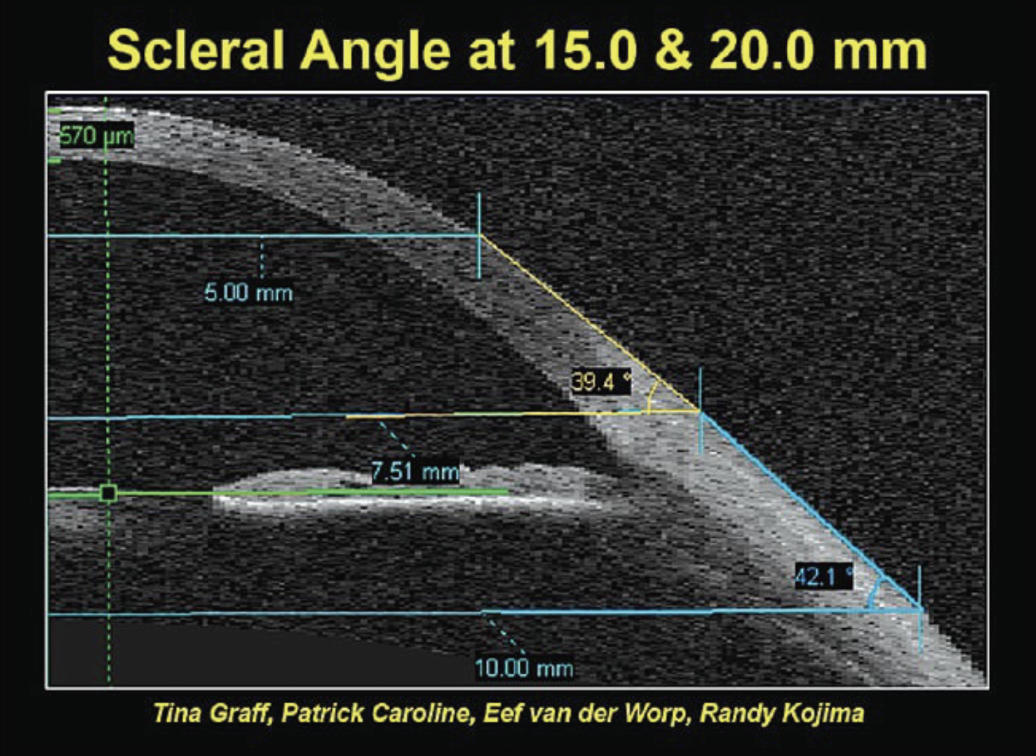 |
|
Fig. 2. Scleral angle variability among 15.0mm vs. 20.0mm chord lengths.1 Click image to enlarge. |
With the 20.0mm chord, the scleral angle variability increased significantly, from 36.6o to 43.2o (Figure 3). This illustrates that the further from the limbus, the more variable the scleral shape becomes. Another study—the Scleral Lens Shape Study—also illustrated large differences in scleral lenses, especially between the nasal region (shallower angle) and the temporal-inferior region (steeper angle; Figure 4). This is also why you may see scleral lenses decenter temporally and inferiorly, especially if they are too large in diameter or have excessive apical clearance.
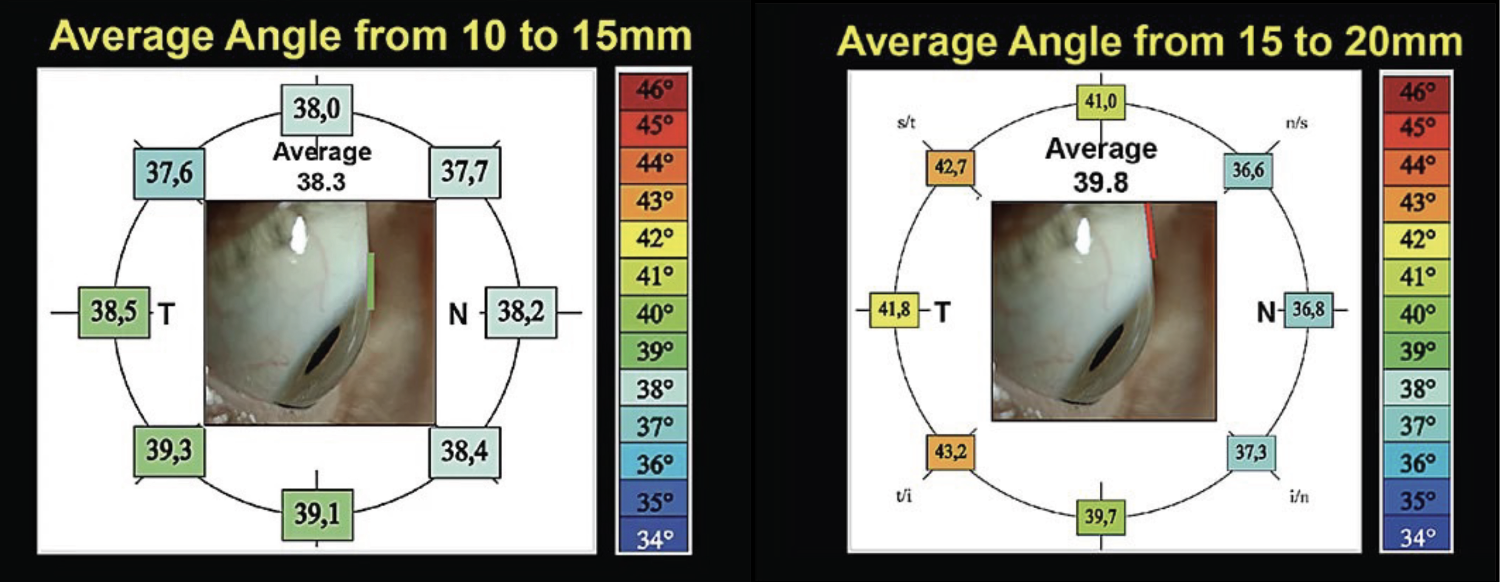 |
| Fig. 3. Among the 15mm chord, scleral angles showed very little variation (left). When compared, the 20.0mm chord showed much more scleral angle variability (right).1 Click image to enlarge. |
This research highlights the need for toric peripheral curves as the size of the scleral lens increases. Imagine what could happen by putting a scleral lens with a spherical back surface on a toric sclera; the asymmetrical scleral lens anatomy can result in a poor fit. In that case, you might see three o’clock and nine o’clock compression or staining and edge lift at six o’clock and 12 o’clock.
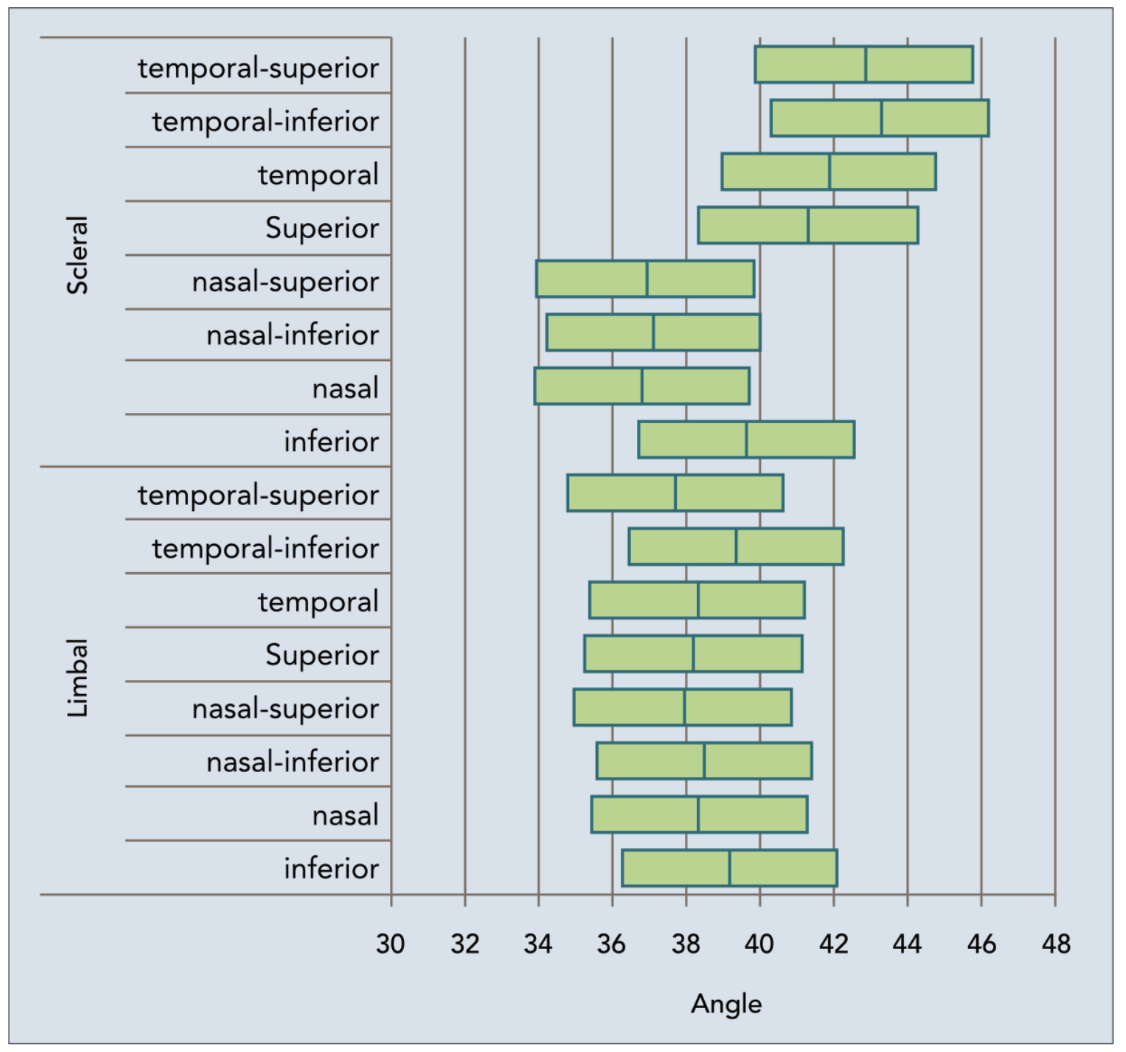 |
| Fig. 4. Variability of scleral lens angle in different meridians.1 Click image to enlarge. |
Double Up the Dyes
Vital dyes are very useful for assessing the ocular surface and contact lens fitting relationship. If you don’t have access to fancy equipment (like how I started), the use of sodium fluorescein (NaFl) and lissamine green (LG) simultaneously has become increasingly valuable in assessing the total ocular surface. We all have been accustomed to adding NaFl to the lens bowl before insertion, which will allow you to see the tear fluid reservoir depth and limbal clearance. However, the addition of LG after the lens is inserted is undervalued. I suggest waiting a minimum of 20 minutes before adding LG. This dye will allow you to assess the lens periphery and can also be used to assess the lens edge if it’s lifting off, influx of the stained fluid in the reservoir (under the lens fogging) or conjunctival irritation and impingement. In my opinion, LG has become just as important as NaFl—if not more—to assess the scleral lens edge because of the high contrast of its green color with the white sclera. See Table 1 below for the different characteristics of NaFl and LG and their uses.
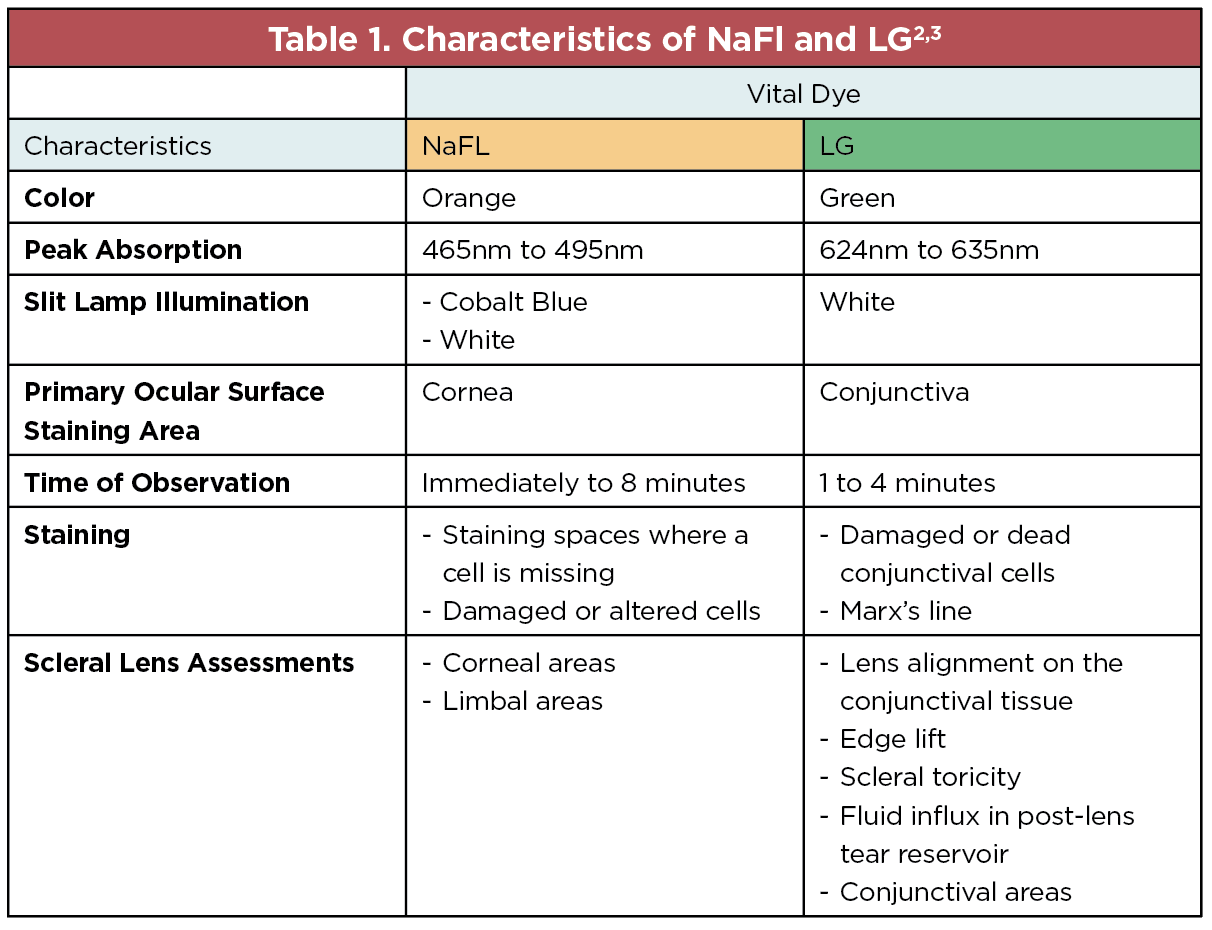 |
| Click image to enlarge. |
After waiting long enough for the scleral lens to settle, I always suggest doing an LG tear uptake test to check the lens edge and potential for fogging. This involves applying a generous amount of LG either directly onto the lens or the lower lid margin. Figures 5 and 6 illustrate the impact LG installation can have on the scleral lens fitting relationship; in Figure 5, a mild amount of LG accumulation is seen inferiorly under the lens, suggestive of potential debris build-up. This may indicate areas where there is a loose edge to tighten the angle. Look along the edge of the lens for areas of LG build-up to indicate areas where the dye could be leaking through. A lens that seems like the perfect fit could have potential for fogging, which may go unrecognized without an LG test.
In Figure 6, you may notice there is a lot more LG accumulating under the lens changing the iris color. This type of fitting would induce a significant amount of fogging within a short time. In addition, pay attention to lens movement. If you see a lot of LG uptake, you should expect to see lens movement with the blink, another hallmark sign that the lens is still too loose. Consider tightening the secondary or peripheral curves to tighten the fit of the scleral lens, which, in turn, will help reduce lens movement and tear exchange under the lens. Another viable modification is switching to a smaller-diameter lens. As shown in Figure 6, the scleral lens becomes more spherical the closer it is to the limbus, allowing it to land more evenly across the sclera, which reduces gabs and edge lift where the tear film could leak in. Large-diameter lenses (≥16.0mm) can likely benefit from adding a toric back surface design.
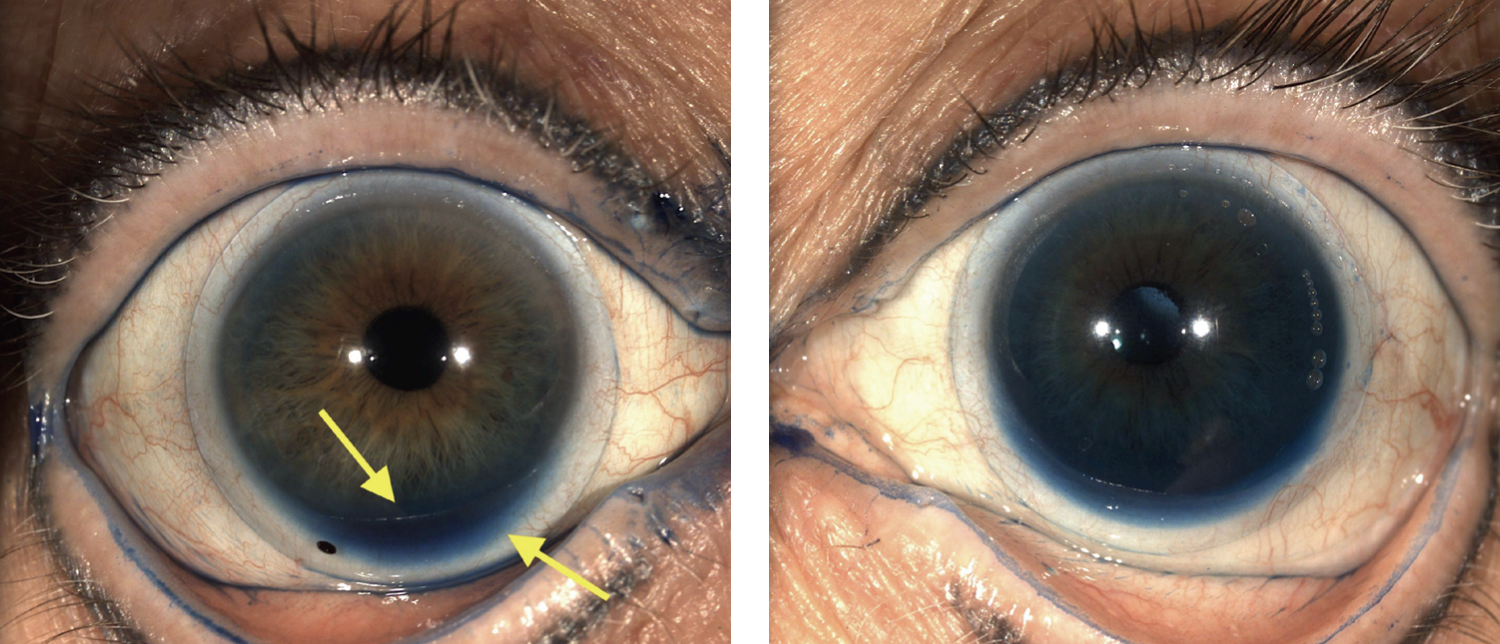 |
| Fig. 5. (Left) Notice the mild accumulation of lissamine green inferiorly along the limbus. Fig. 6. (Right) Notice the large amount of LG throughout, causing the iris to be artificially more blue/green. Click image to enlarge. |
Managing Blanching and Redness
A common challenge scleral fitters will experience from their patients is that they develop redness after wearing the lenses, whether after a few hours or a full day of wear. Edges may look aligned during lens dispensing, but the real test is when patients return for their follow-ups. (Quick tip: Try to schedule these appointments in the afternoon so that lenses have plenty of time to settle.)
When troubleshooting this complaint, it’s critical to evaluate the location and pattern of vessel blanching. If you see circumferential blanching along the lens edge, it could mean the peripheral curve is digging into the tissue and pinching the conjunctiva. This will cut off blood flow in that area, leaving it blanched. A remedy to help reduce blanching along the lens edge is a flatter peripheral curve, allowing the edge to land at an angle better aligned with the contour of the sclera to reduce conjunctival blanching.
Another adjustment that could reduce vessel blanching is flattening the base curve. This will flatten the angles of the entire edge of the lens, but this could impact your central clearance. If you experience asymmetric blanching in scleral lens wearers, meaning certain areas are blanching while other areas have good alignment, this could be due to the anatomical shape of the sclera. A common example is when you see blanching at three o’clock and nine o’clock; this type of fit would benefit from a back surface toric design by modifying the secondary or peripheral curves. Anterior segment OCT (AS-OCT) is another valuable tool to use in these cases, as it allows us to see the lens edge in much greater detail than the naked eye. So, next time you see blanching or redness, see what the AS-OCT shows.
Residual Astigmatism and Front Surface Optics
As much as scleral lenses can provide visual recovery to many patients, residual astigmatism and aberrations may arise, resulting in suboptimal visual performance. This section will share options that may be added to the anterior surface of the scleral lens to optimize vision and restore problems with binocular vision.
The fluid reservoir beneath a scleral lens acts as a refractive lens. Studies have shown that approximately 88% of the anterior corneal astigmatism is equal to the total astigmatism, and the posterior corneal astigmatism will counteract 12% of the anterior corneal astigmatism.2,4-8 The fluid reservoir has a different refractive index compared to the cornea. Dividing the refractive index of the fluid reservoir (1.336) by the refractive index of the cornea (1.376) reveals that 89% of the anterior corneal astigmatism is countered by the fluid reservoir. Therefore, the fluid reservoir overcorrects the total corneal astigmatism by 1%.2,4-8 When doing over-refraction, practitioners can reveal the presence of sphero-cylindrical correction, described as ocular residual astigmatism. This is induced by the posterior corneal surface and the crystalline lens, though it’s thought to mainly result from the latter, which is why it’s known as “lenticular astigmatism.”2,9
The residual astigmatism during a scleral lens fitting may be induced for multiple reasons. It can be due to the fluid reservoir, lens centration, lens warpage or lens flexure. Scleral lenses can exhibit inferior-temporal decentration caused by excessive apical clearance, large diameter, excessive limbal clearance, a toric sclera and weight of the lens. The mechanism can be multifactorial and can be a challenge to identify, altering the lens optics and inducing prismatic effects resulting in residual astigmatism.10,11 Residual astigmatism may persist despite making adjustments to help remove residual cylinder, such as reducing corneal and limbal vault, adding a toric back surface design or changing the diameter, Dk, thickness, design or modulus of the lens. To avoid this, adding a toric anterior surface will correct the cylindrical over-refraction.
Scleral Profilometry and Wavefront-Guided Optics
Adding advanced technology, like scleral profilometry, can help practitioners navigate fitting challenges by providing individualized image-driven guidance. New technology such as scleral topography and profilometry can take the traditional empirical fitting process and instead provide a near-perfectly fit lens that matches the ocular surface on a micron scale. This can help save time and provide optimum comfort and vision for patients. According to the Scleral Lens Shape Study, only about 6% of scleras are simple spheres; therefore, we do not have the big picture of the scleral shape without profilometry.
Irregular corneas can induce higher-order aberrations (HOAs). Studies have shown that eyes with keratoconus can induce five to six times as many HOAs as those with normal corneas.12-14 HOAs can also significantly increase following keratoplasty, especially in eyes that undergo penetrating keratoplasty (vs. deep lamellar endothelial keratoplasty and Descemet’s stripping automated endothelial keratoplasty).15-16 HOAs may also result from the crystalline lens and posterior cornea, which partially compensate for the anterior corneal aberrations in the naked eye. In addition, HOAs may also be induced with lens decentration, giving rise to coma and astigmatism. Customized wavefront-guided scleral lenses can be considered to help reduce HOAs by incorporating wavefront-guided optics to help increase visual acuity and contrast sensitivity. Customized scleral lenses with a customizable HOA front-surface design can be affected by various factors, such as lens decentration, rotation and wettability.17
HOAs can also be influenced by the changes happening in the tear film; studies have reported that variations in the tear volume and fluid dynamics can induce HOAs in regular corneas.18-19 Therefore, it is crucial to manage dry eyes and ocular surface diseases to help reduce unnecessary HOAs.
Adjusting Front-surface Eccentricity
Eccentricity is a measure of how much the deviation of a curve has occurred from the circularity of a given shape. In terms of contact lenses, eccentricity refers to the amount of asphericity in a lens. The higher the eccentricity, the more quickly a contact lens flattens out from the center to the periphery.8 This means the center of the lens will have the most positive power and the power will become increasingly more negative towards the lens periphery.8 This type of geometry was found to induce negative spherical aberration and had a positive impact on visual acuity performance in studies.20-22
Rigid contact lenses with front-surface eccentricity (FSE) improved visual acuity compared to conventional spherical contact lenses.22 A study by Gumus et al. looked at the effects of modifying the FSE in Boston Scleral lenses. Multiple lenses were designed adding FSE of 0.3, 0.6 and 0.8 on patients who have keratoconus, penetrating keratoplasty, post-refractive surgery and ocular surface diseases. The lenses induced a meaningful reduction in HOAs (69% to 77%) in the group with added eccentricity over those that had a spherical front surface design.20 Another study by Hussoin et al., which evaluated vision improvement with the addition of FSE of Boston scleral lenses for corneal ectasia patients, found that an FSE of 0.6 and 0.8 had noticeable improvement in high-contrast and low-contrast visual acuity.21 Furthermore, Mahadevan et al. showed the impact FSE can have on improving visual acuity of 17 patients with corneal ectasia; in that study, patients with FSE of 0.6 had significant improvement in their vision compared to the group without eccentricity.22 This type of geometry added to scleral lenses has shown the impact FSE can have on inducing negative spherical aberration and reducing HOAs, ultimately helping to improve visual acuity.
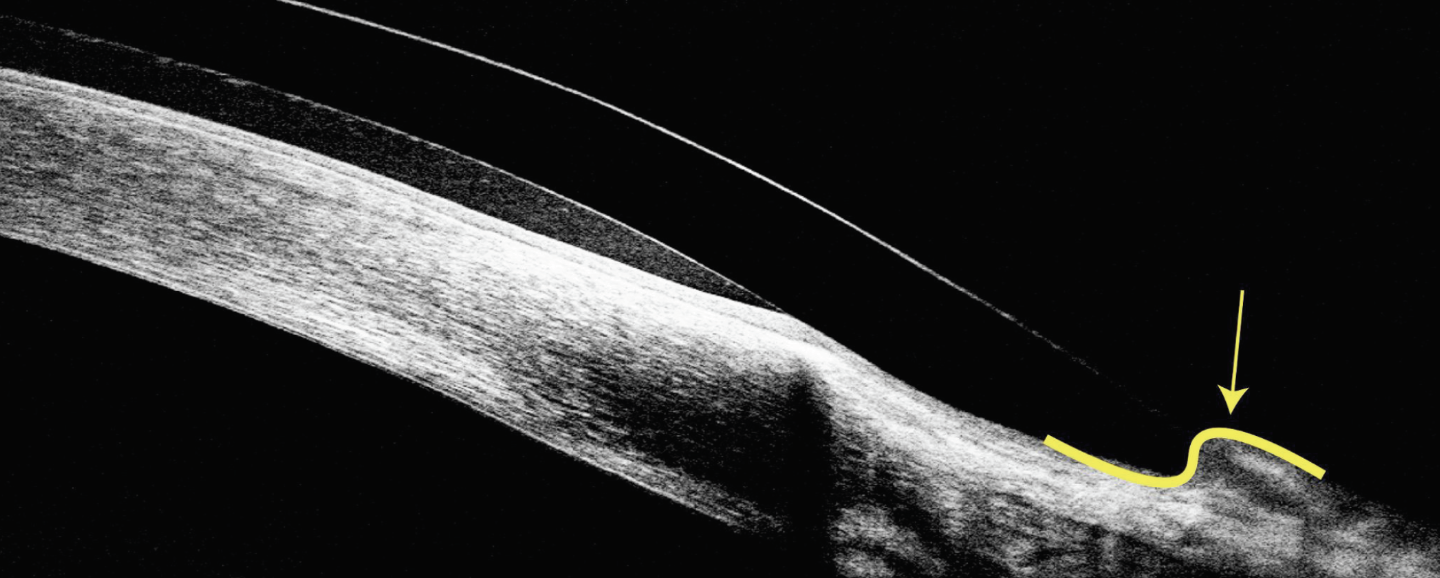 |
| Fig. 7. This steep peripheral curve has caused the conjunctiva to start riding over the anterior surface of scleral lens. Click image to enlarge. |
Prolate vs. Oblate Designs
There are two general designs of scleral lenses: prolate and oblate. The former describes a design in which the back optic zone radius is steeper than the peripheral radius. Conversely, an oblate design has a back optic zone radius flatter than the peripheral radius. Prolate designs are generally indicated in all forms of keratoconus and ectasias, while oblate designs are generally designed for cases in which the cornea is flatter centrally compared to the periphery, such as post-surgical for myopia or radial keratotomy. Fitting a prolate lens on an oblate cornea the lens can create excessive clearance centrally and can create touch in the mid-peripheral cornea.
Excessive central clearance can reduce oxygen transmissibility to the cornea, create lens decentration, fogging and reduced vision quality due to the prismatic effect of a decentered ectasia.23 Oblate designs will help create a proper vault in the mid-peripheral cornea while maintaining the appropriate central clearance. Fitting an oblate design on a prolate cornea can have advantages because the peripheral cornea is steeper than the central. This allows for adequate vault over the decentered ectasia. Furthermore, the central lens area will be closer to the cornea, resulting in greater oxygen delivery, reduction of the lens minus power, a larger optic zone diameter and better on-eye stability.23
Takeaways
Scleral lens designs continue to expand and improve to meet ever-increasing demand for them. When making a life changing impact to someone’s sight with scleral lenses - it should not be quick or a ‘one-size fits all’. Understanding that the sclera becomes more toric the further beyond the limbus is important. If you are using a scleral lens larger than 15.0mm, look at the relationship between secondary and peripheral curves on the ocular surface as carefully as possible. Use a combination of the tools listed above to help you improve your fitting success and efficiency.
Dr. Asnaashari graduated in 2018 from the University of California Berkeley School of Optometry. He currently works full time in private practice and owns Arden Park Optometry in Sacramento, CA. After finishing school, he organically developed an emphasis in the areas of myopia management and custom contact lenses for patients with corneal disorders. He is a fellow of the American Academy of Optometry and Scleral Lens Education Society. He is currently serving as President of his local optometric society, the Sacramento Valley Optometric Society. He has no financial disclosures.
1. Kojima R, Caroline PJ, Graff T, et al. Eye shape and scleral lenses. Cont Lens Spectrum. 2013;4:38-43. 2. Fadel D. Scleral lens issues and complications: their recognition, etiology, and management. Dougmar Publishing Group Inc. 2020. 3. Dry eye redefined: TFOS DEWS II report. TFOS. Published July 17, 2017. Accessed June 15, 2023. www.tfosdewsreport.org. 4. Born ME. Wolf principles of optics. Pergamon Press. 1980;6:188-9. 5. Illueca C, Mas D, Perez J, et al. Refractive analysis of the human cornea through propagated fields. J Mod Opt. 2001;48(5):811-29. 6. Pérez J, Mas D, Illueca C, et al. Complete algorithm for the calculation of light patterns inside the ocular media. J Mod Opt. 2005;52(8):1161-76. 7. Pérez J, Mas D, Miret JJ, et al. Fresnel-based analysis of Kasprzak’s crystalline model: statistical results and individual predictions. Opt Int J Light Electron Opt. 2005;116(2):49-57. 8. Douthwaite WA. Contact lens optic and lens design. 3rd ed. Elsevier Health Sciences. 2006;7:202-51. 9. Piñero DP, Ruiz-Fortes P, Pérez-Cambrodí RJ, et al. Ocular residual astigmatism and topographic disparity vector indexes in normal healthy eyes. Cont Lens Anterior Eye. 2014;37:49-54. 10. Gumus K, Gire A, Pflugfelder SC. The impact of the Boston ocular surface prosthesis on wavefront higher-order aberrations. Am J Ophthalmol. 2011;151:682-90. 11. Kerns RL. Clinical evaluation of the merits of an aspheric and bispherical cortact lens for patients manifesting residual astigmatism. Am J Optom Physiol Opt. 1974;51:750–7. 12. Negishi K, Kumanomido T, Utsumi Y, Tsubota K. Effect of high-order aberrations on visual function in keratoconic eyes with rigid gas permeable contact lens. Am J Ophthalmol. 2007;144:924-9. 13. Pantanelli S, MacRae S, Jeong TM, Yoon G. Characterizing the wave aberration in eyes with keratoconus or penetrating keratoplasty using a high-dynamic range wavefront sensor. Ophthalmology. 2007;114:2013-21. 14. Applegate RA, Hilmantel G, Howland HC, et al. Corneal first surface optical aberrations and visual performance. J Refract Surg. 2000;16:507-14. 15. Hindman HB, McCally RL, Myrowitz E, et al. Evaluation of deep lamellar endothelial keratoplasty surgery using scatterometry and wavefront analyses. Ophthalmology. 2007;114:2006-12. 16. Muftuoglu O, Prasher P, Bowman RW, et al. Corneal higher-order aberrations after Descemet’s stripping automated endothelial keratoplasty. Ophthalmology. 2010;117:878-84. 17. Marsack JD, Ravikumar A, Nguyen C, et al. Wavefront-guided scleral lens correction in keratoconus. Optom Vis Sci. 2014;91:1221-30. 18. Goto E, Ishida R, Kaido M, et al. Optical aberrations and visual disturbances associated with dry eye. Ocul Surf. 2006;4:207-13. 19. Lin YY, Carrel H, Wang IJ, et al. Effect of tear film break-up on higher order aberrations of the anterior cornea in normal, dry, and post-LASIK eyes. J Refract Surg. 2005;21:525-9. 20. Sabesan R, Johns L, Tomashevskaya O, et al. Wave-front guided scleral lens prosthetic device for keratoconus. Optom Vis Sci. 2013;90;4:314-23. 21. Hussoin T, Le HG, Carrasquillo KG, et al. The effect of optic asphericity on visual rehabilitation of corneal ectasia with a prosthetic device. Eye Contact Lens. 2012;38:300–5. 22. Fadel D. Three of a kind. PentaVision. December 15, 2023. Accessed June 15, 2024. clspectrum.com/issues/2023/july/three-of-a-kind. 23. Mahadevan R, Arumugam AO, Madhumathi, et al. Clinical visual performance of different front surface eccentricity in PROSE. Cont Lens Anterior Eye. 2012;35;S1:e6–7. |


