 |
Tackling Corneal Transplants in Clinical Practice
A comprehensive understanding of these procedures is critical to ensure optimal patient care and outcomes.
By Aaron Bronner, OD
Release Date: November 15, 2022
Expiration Date: November 15, 2025
Estimated Time to Complete Activity: 2 hours
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group.
Educational Objectives: After completing this activity, the participant should be better able to:
Recognize the various corneal transplant options.
Make appropriate referrals for these patients.
Educate and support their corneal transplant patients.
Effectively comanage corneal transplant patients with ophthalmologists.
Participate in the postoperative care of corneal transplants.
Target Audience: This activity is intended for optometrists engaged in managing corneal transplant patients.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by PIM and the Review Education Group. PIM is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education and the American Nurses Credentialing Center to provide CE for the healthcare team. PIM is accredited by COPE to provide CE to optometrists.
Reviewed by: Salus University, Elkins Park, PA
Faculty/Editorial Board: Aaron Bronner, OD
Credit Statement: This course is COPE approved for 2 hours of CE credit. Activity #124924 and course ID 81404-PO. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements: PIM requires faculty, planners and others in control of educational content to disclose all their financial relationships with ineligible companies. All identified conflicts of interest are thoroughly vetted and mitigated according to PIM policy. PIM is committed to providing its learners with high-quality, accredited CE activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of an ineligible company.
Those involved reported the following relevant financial relationships with ineligible entities related to the educational content of this CE activity: Authors—Dr. Bronner has no financial interests to disclose. Dr. Sicks receives fees from Alcon. Managers and Editorial Staff—The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
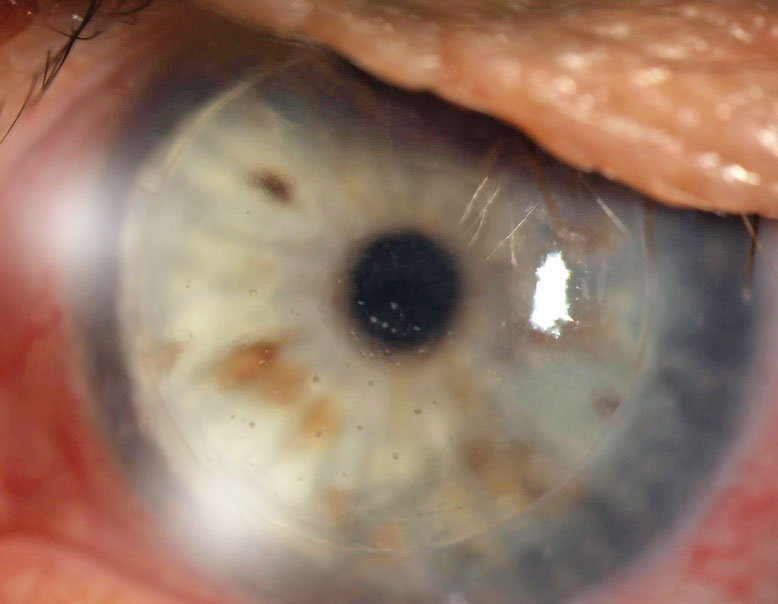 |
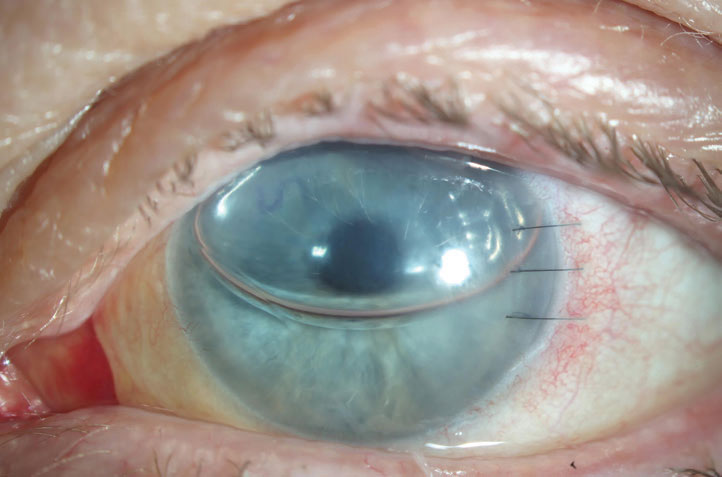
| Fig. 1. This PK is suffering from an endothelial rejection. This can be visualized as KP scattered across the endothelium of the graft. The episode was successfully treated, but the patient’s graft failed two years later likely as a result of this rejection episode. Click image to enlarge. |
Corneal transplants are the oldest and most successful solid tissue transplants in medicine.1 Despite the frequency with which these are performed, many ODs feel daunted when encountering a transplant in the clinic, especially if there is a concern with the health of the graft. This discomfort may be due to a lack of clear didactic education on transplant science, the relatively high risk of complications and the wide array of transplant procedures available.
Developing a solid grasp of transplants starts with looking at the overarching themes that govern the behavior of all transplants and then applying those rules to the specific transplant you are dealing with. First, a deep understanding of the immunology of transplants, specifically the role the transplant plays in modulating immune responses, is critical.
In a penetrating keratoplasty (PK), the most important layer is the transplanted endothelium. Understanding its importance and influence on graft survival, both in the presence and absence of rejection, is the second key. Finally, understanding the optical and immunologic effects of sutures on the transplant can help you better manage patients in the postoperative phase.
With a solid understanding of these three broader topics, the behavior of all the currently available transplants, as well as any future transplants, can be easily understood. Cutting edge advances in transplantation—for example, decellularized animal tissue as a transplant—are best understood with this background knowledge of corneal graft immunology.
Key 1: Immunology of Transplants
Understanding how the host immune system responds to the presence of foreign graft material helps predict rejection risk and graft survival. There are several broad types of transplants: xenografts (a transplant from one species to the next), allografts (a transplant from one member of the same species to a different individual) and autografts (a transplant from another location on the patient’s own body).
In general, xenografts have extremely high rates of immunologic reaction and aren’t used, barring acellular tissue transplants. Allografts, which may be either HLA-matched or unmatched (as is the case with corneal transplants in the United States) from donor to host, carry a risk of rejection that varies with the organ or tissue transplanted. Autografts have no risk of immunologic rejection. Nearly all corneal transplants are allografts, though the most common limbal transplant, simple limbal epithelial transplant, is an autograft from one eye to the other.
Within an organ or solid tissue transplant, the immune response is only able to identify graft cells (via HLA-antigens) as foreign, and so only transplants containing cells can stimulate a rejection episode. This is why xenografts of animal connective tissue/collagen can be used without risk of rejection.1 In a full-thickness corneal transplant, the targets of rejection are the epithelium, keratocytes and endothelium.
The epithelium makes up the bulk of the antigenic cellular load; however, epithelial cells are fully replaced by host cells via limbal replenishment (and the limbus falling outside the margins of the graft) within the early months postoperatively.2,3 Therefore, the epithelium is only a target of rejection for a short window. The longevity of keratocytes is in question, but they may persist for up to five years (though some estimates are much shorter).
Keratocytes express little HLA-antigen and are the least antigenic of transplanted corneal cells.2,3 The corneal endothelium persists indefinitely, thereby the endothelium always remains a possible target for rejection. Compounding this is the non-miotic nature of the endothelium. Unlike the epithelium or keratocytes, which can be replaced with new host cells if affected by rejection, the donor endothelium affected by an immune response does not regenerate. If a case of endothelial rejection is severe or treatment is delayed, the endothelial pump mechanism can collapse, causing corneal decompensation and edema. As such, endothelial rejection (in grafts containing endothelium) is a primary indication for repeat transplant in these eyes.4-6
Since rejection is a clinical manifestation of the immune system’s response to the cells of a transplant, white blood cells (WBCs) will be present within the graft. Vascularization may develop with or without rejection, but graft vascularization alone (though increasing the risk of rejection) is not a strong indication of active rejection.7 As there are three different cellular targets for rejection, there are a few different common ways transplant rejection will manifest.8,9
If epithelial rejection occurs in the short few-week window following a transplant, it will manifest as a gray edematous zone of epithelial tissue across the graft. Ultimately, there will be sloughing of the rejected donor epithelium. The host limbus will then fill in this zone with host epithelium. This is a non-terminal form of rejection, but it does mean the host immune system has acquired a sensitivity to the graft and a more severe form of rejection may subsequently develop.8,9
 |
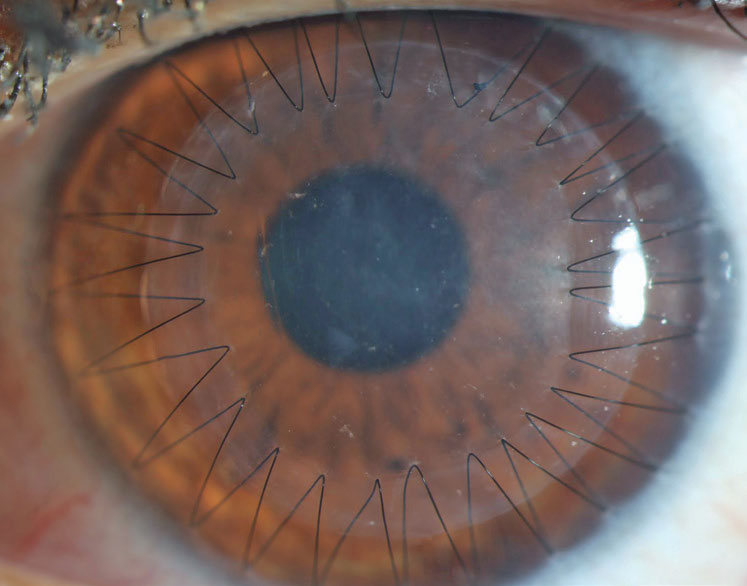
| Fig. 2. Sutures and trephination of both the host and donor tissue introduce the possibility of high and irregular astigmatism. This may be mitigated with specialty contact lenses or procedures, including CRIs or PTK, that also slow down the visual rehabilitation of the transplant. Click image to enlarge. |
Rejection involving the keratocytes leads to stromal rejection. Usually, stromal rejection manifests as nummular lesions in the stroma as WBCs cluster around donor keratocytes—similar in appearance to the nummular keratitis seen in herpes zoster ophthalmicus and the somewhat larger and deeper subepithelial infiltrates often seen following adenoviral infection. Occasionally, acute stromal rejection will occur, appearing as sudden and prominent corneal edema without evidence of WBCs. As with epithelial rejection, stromal rejection seldom leads to optical failure of the transplant but may mean a more severe form of rejection could develop.8,9
Endothelial rejection manifests as diffusely distributed keratic precipitates (KP), usually with overlying edema. Occasionally, a linear migratory front of KP known as a Khodadoust line forms. Both forms are typically accompanied by substantial corneal edema. Endothelial rejection may lead to graft failure via immune-mediated collapse of the endothelial pump and subsequent corneal edema (Figure 1).8,9
Primary treatment of any corneal graft rejection consists of topical corticosteroids (start at Q1h dosing initially and observe the response over a week). Oral corticosteroids and periocular injections of steroid and topical compounded immunomodulatory agents (tacrolimus 0.03% and cyclosporine 0.5% to 2.0%) can all be used to supplement, but not replace, topical steroids in severe/high-risk cases.10-12
Corneal edema within a transplant is both a frequent manifestation of rejection and the end result of endothelial graft failure (whether due to rejection or exhausted lifespan). Any endothelium-containing transplant with new-onset edema should be presumed to be amidst a rejection episode and thus treated with increased dosing of topical corticosteroids.4 Resolution of edema and the ability to visualize KP confirms an endothelial rejection. Resolution of edema without underlying KP would confirm acute stromal rejection. If the edema fails to clear over a few weeks, this would confirm failure of the graft and need for a subsequent transplant.
This brief overview of rejection and graft immunology highlights two key pearls for practitioners. First, grafts containing endothelium carry an indefinite risk for rejection, as donor endothelium is never replaced by host tissue. Second, any rejection of transplanted endothelium can induce graft failure. Thus, grafts containing endothelium need long-term topical steroid therapy to prevent rejection.
Key 2: Influence of Endothelium
The transplanted endothelium always limits the lifespan of a corneal graft, even in the absence of rejection. Since the transplanted endothelium carries prolonged potential for graft rejection, endothelial rejection is the only common way rejection leads to graft failure (via collapse of the endothelial pump).
Endothelial cell density reduces as we age, a trend which is accelerated when the endothelium is transplanted. Up to 40% of graft endothelium can be lost simply due to the surgical process, and further decompensation occurs over time.4,13,14 Assuming an uneventful recovery, this accelerated decompensation (primarily caused by trauma from the surgery itself) will lead to a finite life expectancy of any graft containing endothelium of, on average, between 10 and 20 years (though outliers exist).4
Once the graft endothelium reaches the end of its functional life, the graft becomes edematous and a new transplant is needed. Although this process will proceed in the absence of rejection, cases of endothelial rejection that are successfully treated and result in clearing of the cornea will still result in further reduction in endothelial cell density and thus a shorter graft lifespan. Anytime the endothelium is transplanted, the patient should be aware of the graft’s average life expectancy and the potential necessity of subsequent re-graft procedures.
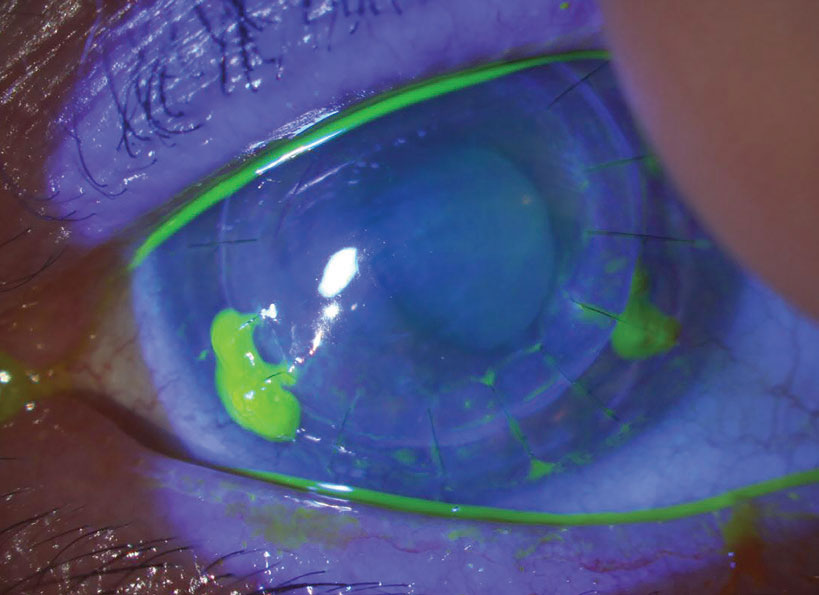 |
| Fig. 3. Loose/exposed sutures pick up fluorescein dye. In this example, sutures at five, seven and eight o’clock are all loose and should be removed. Late in the postoperative course, this can be done with low risk of any problem. In the early postoperative period, however, given that you’d be removing adjacent sutures at seven and eight, the patient should be referred back to the surgery center, as they may need that zone reinforced with a new suture. Click image to enlarge. |
Key 3: Effect of Sutures
The process of suturing select corneal transplants into place is a primary cause of astigmatism that can affect a patient’s refractive outcome. The average topometric astigmatism following PK is between 4.00D and 6.00D, with 20% of patients having over 8.00D even following suture removal.15,16 The astigmatism is often irregular, which leads to frequent reliance on specialty contact lenses fit postoperatively to achieve good vision.
The need for contact lenses postoperatively highlights two main considerations: (1) intolerance to lenses is a poor indication for proceeding to a transplant, as the patient may end up back in specialty lenses following surgery and (2) elderly patients may have dexterity limitations that make specialty lenses a challenge, thus creating a substantial barrier to achieving good vision after a PK (Figure 2).
Sutures can be adjusted or removed to modify both the magnitude of toricity and the degree of irregularity postoperatively; however, the effectiveness of these manipulations is not predictable and can vary significantly from patient to patient. While limited interrupted suture removal can begin as early as weeks following surgery, widespread removal cannot occur prior to the graft being fully healed. This takes several years following transplant for a PK, though with a deep anterior lamellar keratoplasty (DALK), sutures can generally be removed in about half the time. Determining total healing is difficult and involves a somewhat subjective assessment of fibrosis of the deep graft-host interface. This decision is best left in the hands of the operating surgeon.
Following targeted suture removal or adjustment, or full suture removal, if high astigmatism persists, further reduction of astigmatism can be performed with phototherapeutic keratectomy (PTK), corneal relaxing incisions (CRIs) or compression sutures. These procedures often take weeks to months to stabilize, which can further delay achievement of best-corrected vision. On average, it takes two to five years for a PK patient to stabilize, with longer times for patients who require further surgical procedures to reduce astigmatism (Figure 3).17
Corneal sutures induce obvious effects on vision and optical quality, but they also create a subtle yet important effect on corneal immunology. The body responds to the placement of corneal sutures by immediately upregulating all VEGF isoforms.18,19 This results in immediate corneal neovascularization and lymphangiogenesis at the margins of the graft, a process which obviously erodes the immune privilege of the graft. This upregulation of VEGF is short-lived, and the vessels created in this period regress; however, studies have shown that despite the endothelial graft antigen persisting indefinitely, rejection is most likely to occur in the first several months following surgery—a fact that indicates this early upregulation of VEGF may be important.20-22
Sutures not only impact the early immune response but also create a lasting pro-inflammatory stimulus, where mechanical irritation leads to recurrent suture infiltrates at the peripheral bite of the suture (Figure 4). These infiltrates create photophobia and discomfort but, more importantly, in bringing the immune system to the margins of the graft, they increase the risk of subsequent rejection. Fortunately, these infiltrates also respond well to topical corticosteroids. If recurrent, however, the suture needs to be removed as soon as is feasible.
In summary, much of the challenging recovery associated with a corneal transplant—highlighted by a slow visual recovery, high amounts of irregular corneal astigmatism and subsequent need for a specialty contact lens—can be attributed to the presence of corneal sutures. The sutures also negatively affect corneal immunology by increasing the risk of rejection and the severity of that rejection.
When you put these key concepts together in general transplant immunology, considering the importance of the endothelium, and include the influence of sutures on immunology, one thing is clear: a graft that both contains endothelium and is sutured in place creates the greatest erosion of immune privilege and has the greatest risk of rejection and risk of that rejection leading to failure. These transplants have the greatest need for ongoing topical corticosteroid therapy. This carries with it the potential side effects of chronic corticosteroid use, including increased risk of glaucoma, cataract and rejection. Should any of these adverse events occur, their treatment and prognosis are worse in the setting of a transplant than if encountered alone.
Finally, even if an endothelium-containing transplant successfully avoids rejection and steroid-related side effects, the grafted endothelium limits the life expectancy of that graft to approximately 10 to 20 years, and it will eventually need to be replaced.
So, what types of transplant satisfy all of these features (sutured and endothelium transplanted)? PK alone. Newer lamellar transplants improve on one or both of these risk factors resulting in reduced risk of rejection, an improved visual recovery or both. As we review each specific transplant type, based on the tissues that are transplanted, consider the influence of the endothelium and sutures on the anticipated recovery of that graft.
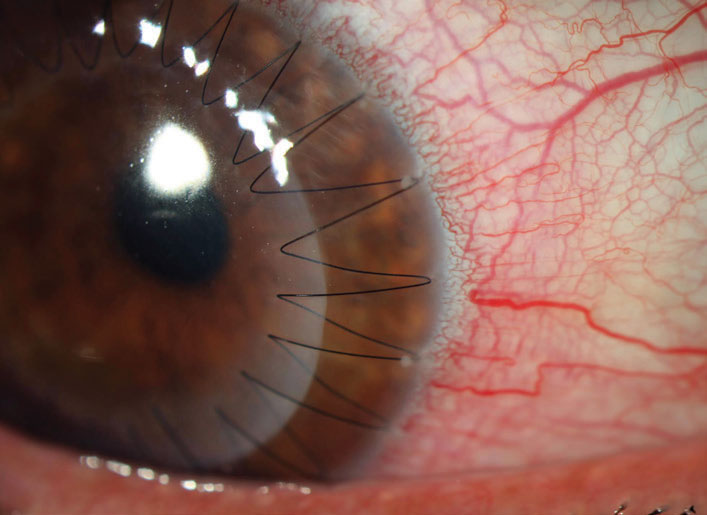 |
| Fig. 4. Small infiltrates can be seen at the peripheral edges of the half past one, two and three o’clock suture bites. These respond well to corticosteroids and don’t represent a rejection episode but, given that they bring the immune system into proximity with the graft, they may increase the risk of rejection. Click image to enlarge. |
PK Ins and Outs
This procedure was first successfully performed in 1903 by Dr. Edward Zirm on a patient with an alkaline burn.1 The procedure itself was notable as it was performed prior to eye banking, modern suture material (only cat gut and silk were available), the discovery of penicillin—and thus antibiotics—and the pharmacologic use of glucocorticoids. It’s truly remarkable that a PK was able to overcome these limitations, illustrating just how well the cornea is positioned to accept allografts when compared with other tissues and organs.
A PK involves full-thickness transplantation of all layers of the central cornea and was the only widely available transplant option for nearly a century. Of all corneal transplant techniques, PKs disrupt the normal anatomy, optics and immunology of the cornea the most; therefore, they have the lengthiest and most fraught postoperative recovery period. While its indications have dwindled with the development of more targeted lamellar transplants, PKs still account for approximately 35% of corneal transplants performed in the United States today.1
During a PK, the graft button is usually 7.5mm to 9.5mm in size (depending on the host’s native corneal diameter), and the host is trephined (cut in a circular fashion) in a slightly smaller diameter (0.25mm).23 Larger transplants are associated with better optical outcomes, with the influence of irregularity induced by suture tension diminishing the further away from the visual axis. This should be tempered by the fact that larger grafts (those closer to the host limbus) are associated with higher rates of rejection and other unwanted immune-related sequela.24 The central cornea maintains a state of immune privilege, but that privilege wanes further out toward the limbus. Once the graft is secured by sutures, which may be “running” or “interrupted” (though all grafts receive at least four intraoperative interrupted sutures), the patient is sent home to begin the recovery process, the visual component of which can take years.17,23
With knowledge of the influence of sutures and transplanted endothelium on risk and recovery, it is easy to predict that, on average, PKs carry the slowest visual recovery, the greatest risk of steroid-related side effects and the greatest risk of rejection leading to failure. Even with these limitations, a PK is the only surgical option for any full-thickness pathology and is generally felt to be the most straightforward transplant surgery. Despite the availability of deep anterior lamellar keratoplasty (DALK), Descemet stripping automated endothelial keratoplasty (DSAEK) and Descemet membrane endothelial keratoplasty (DMEK) for various indications, PK is still the second most common transplant technique in the United States, likely owing to its surgical “simplicity”—at least relative to other options.24
DSAEK, DMEK Deep Dive
Endothelial keratoplasty has its roots in Charles Tillet’s attempt in the 1950s to suture in a posterior lamellar transplant. After this early attempt failed, the field was left fallow for decades. It wasn’t until Dr. Garrit Melles’s early work in the 1990s, followed by adjustments in technique by Mark Terry, Mark Gorovoy, Francis Price and others, that we were able to arrive at the two modern iterations of posterior lamellar transplants: DMEK and DSAEK.25 By the time Descemet stripping endothelial keratoplasty (DSEK), a predecessor to DSAEK, arrived in the early 2000s, these endothelial procedures almost immediately replaced PK as the graft of choice for endothelial disease. In 2005, deep lamellar endothelial keratoplasties (DLEK), the only available posterior lamellar graft at the time, accounted for 4.5% of transplants in the United States, compared with 2010 when DSEK (more recently developed) accounted for 40% of all corneal transplants in the country.26
Widespread adoption of the techniques among surgeons was driven by several factors. Endothelial disease is a primary indication for corneal transplantation, and the availability of targeted endothelial transplant options carries several recovery advantages over PK. By eliminating the securing sutures used in PK, endothelial keratoplasty achieves rapid recovery of vision relative to PK and a very modest and predictable refractive effect, does not create irregular astigmatism and has a smaller influence on corneal immune privilege, leading to less rejection and less rejection-induced graft failure.
When the technically complicated DLEK advanced to DSEK, the process was simplified and could be adopted by more cornea specialists. The more tedious and delicate DLEK required the surgeon to perform a posterior dissection of the host stroma and was the domain of the handful of surgeons who dedicated their careers primarily to corneal transplants whereas this step was abandoned with DSEK.25
 |
| Fig. 5. This typical DMEK one-day postoperative appearance has a large gas bubble present in the anterior chamber. When the patient is supine, the gas bubble will press the donor graft into place. The bubble will limit vision to 20/400 or worse until it reabsorbs out of the visual axis. An “S” stamp, which can be placed by the eye bank upon surgeon request, can also be seen, indicating the graft is in the correct orientation. An inverted “S” would mean the transplant is upside down. Click image to enlarge. |
DSAEK and DMEK are similar modern procedures for endothelial transplantation. During surgery, the host Descemet membrane and endothelium are removed. The 8.5mm to 9.0mm graft is then inserted into the anterior chamber, unfolded and positioned centrally. It is then supported with a gas bubble (depending on the surgery center, this bubble may be air or a high-density gas mix), and the patient is sent home with supine positioning restrictions over the first several days postoperatively to allow the air bubble to press the graft into place (Figure 5).
Both of these transplants have some risk of graft dislocation in the first week following surgery. Dislocation may require a repeat bubble or even repeat transplant (usually after a repeat bubbling fails). Thus, the first several days following a DSAEK or DMEK carry the greatest risk of complications. If a patient makes it out of this time period without complications, the visual recovery is usually smooth over the next three to six months.
DSAEK is more widely performed than DMEK, for purely mechanical reasons.23 DMEK has slightly superior visual outcomes, more rapid time to visual stability and a significantly lower risk of rejection (which is paradoxical considering there isn’t much difference in theoretic antigenicity between the two graft types); however, due to scrolling of the DMEK graft as it is placed in the eye, it is a more challenging procedure with greater risk of graft dislocation and early graft failure. For these reasons alone, DSAEK is the more frequently performed of the two surgeries.23
Since endothelial transplants erode immune privilege less then PK, the rate of rejection is smaller as is the severity of rejection. These procedures also respond better to medical therapy resulting in less frequent failure.27 However, although both of these endothelial grafts have a lower risk of rejection than PK, the risk isn’t eliminated altogether.27
DALK Discussion
Clinically, a DALK appears just like a PK and has the same sutures that limit PKs, but it also carries some significant advantages over a PK. A DALK procedure transplants all tissue anterior to the endothelium and is used for keratoectasia, stromal dystrophies and non–full-thickness corneal scarring. The anterior graft is sutured into place like a PK and, also as with a PK, the securing sutures create a number of optical and immunologic consequences. However, leaving the host Descemet membrane and endothelium in place promotes faster healing and better tectonics, which leads to quicker optical stability. Further, the importance of the immunologic influence of sutures is blunted by the fact that the endothelium is not transplanted, so there is close to no long-term risk of rejection leading to failure. This allows a more rapid elimination of topical steroids which limits the risk of steroid-related side effects.28,29
Despite these advantages and the surgery’s relatively wide set of indications, DALK is by far the least frequently performed transplant surgery.23 This is primarily due to the demanding nature of the surgical procedure itself. Achieving a dissection plane down to the Descemet membrane without perforating it is a challenging skill. In many cases, the tissue is damaged or torn in the dissection phase. If the tears are large enough, the procedure may need to be aborted and conversion to a PK can be required. On the other hand, if dissection is not deep enough (to the level of the Descemet membrane), a scarred interface may occur, limiting the visual outcome. For this reason, DALKs have a slightly worse average postoperative best-corrected visual acuity compared with PKs (Figure 6).30
Decellularized Transplants
A recent publication in Nature Biotechnology highlighted the potential for bioengineered porcine dermal collagen to mimic the human cornea. The publication resulted in a number of popular media articles suggesting (perhaps prematurely) that such corneal transplants could restore vision in patients with keratoconus.
After being heavily processed, porcine collagen from either pig skin or eyes can be decellularized (killing the cells) and transplanted into patients with keratoconus who are too far advanced to crosslink and losing the ability to achieve good vision with their contact lenses. By placing this acellular tectonic graft in a stromal pocket, the risk of rejection is eliminated, progression of the disease stops (and may even slightly regress) and there is minimal impact on refraction since there are no sutures.31,32
The concept is similar to Bowman’s layer (BL) transplants, but decellularized animal transplants have the added benefit of not requiring human tissue, which would increase access to tissue in countries without eye banking capability sufficient to meet the needs of the population.
DALK Case StudyLet’s use a case to illustrate the advantages of this procedure. A 20-year-old patient with keratoconus plans to undergo bilateral PK. We anticipate frequent and sustained dosing of steroids, which increases the risk of glaucoma, cataract and infection. Further, even barring a rejection episode, that graft will likely fail due to endothelial decompensation by around the time the patient is 40. At that point, a DSAEK or DMEK may be attempted under the failed PK. With an uneventful recovery, that endothelium will likely fail by the time this patient is 60, requiring yet another transplant. The graft is then repeated every 10 to 20 years. If the patient initially underwent DALK, they would have less need for topical steroids and a lower risk of rejection, and they would keep their own endothelium, which will likely last the rest of their life. This is in comparison to the predicted three to four transplants a young PK patient may need during their lifetime. While DALK has a lot of surgical challenges, it carries such substantial long-term advantages. Its use should at least be considered for patients with anterior pathology requiring transplant. |
DWEK Dos and Don’ts
Descemetorhexis without endothelial keratoplasty (DWEK) or Descemet stripping only is a procedure exclusive to those with Fuchs’ endothelial corneal dystrophy (FECD) and best suited for those with heavy centralized guttata. In FECD, guttata can affect vision even in the absence of edema if they accumulate heavily in the visual axis. Central guttata also put the surrounding endothelial cells on stretch, which is thought to induce apoptosis, thereby speeding up the process of decompensation. Guttata also prevent the migration of healthy endothelium into spaces of endothelial cell loss—a normal step in endothelial cell injury.
DWEK involves stripping 3.0mm to 4.0mm of the central Descemet membrane, endothelium and accompanying guttata. After the procedure, the patient’s peripheral endothelium may be able to fill that empty space following normal migration and removal of the deleterious effects of the guttata. Of course, these patients will suffer from prominent corneal edema until this process is completed, so the visual recovery can be quite slow.
Advantages of this procedure are that it does not require transplant tissue (reducing both cost and the risk of rejection) and eliminates the need for supine positioning postoperatively that comes with DMEK and DSAEK. The procedure is better suited for younger patients with central guttata.33,34
BL Transplant
This is the acellular layer of connective tissue immediately posterior to the epithelial basement membrane. Its role has not been fully elucidated, but it is thought to be primarily tectonic. Patients with keratoglobus, for example, are believed to have an aberrant BL.35 Surgeons in Europe have experimented with transplanting donor BL into a stromal pocket in a host with severe keratoconus.36 The use of a tectonic acellular graft avoids immunologic risks of rejection while at the same time halting progression of the disease and possibly inducing modest regression.
The target population for this procedure is relatively small. Certainly, corneal crosslinking is the procedure of choice for most patients with progressive keratoconus, and when the disease is too advanced for success with specialty contact lenses, we should consider a transplant. However, patients who are successful contact lens wearers, yet too thin for crosslinking, may be prime candidates for BL transplantation in order to avoid more traditional keratoplasty and continue with their contact lens wear.
Ultrathin DSAEK
The difference between DSAEK and DMEK is the inclusion of posterior stroma to the DSAEK graft. DMEK grafts contain only Descemet membrane and endothelium. The inclusion of posterior stroma in DSAEK insulates the endothelium from intraoperative damage and makes the graft easier to handle and place than DMEK. The elasticity of DM without stroma causes DMEK grafts to scroll, which can be difficult to open during surgery. They often want to scroll at the edges postoperatively, leading to a more difficult surgery and a graft that is more likely to detach earlier on. The lack of posterior stroma in DMEK is also speculated to be a significant contributor to the superior optical outcomes of DMEK (no hyperopic shift like in DSAEK and greater odds of achieving best-corrected acuity of 20/20).
To achieve the optical benefits of DMEK while retaining the surgical benefits of DSAEK, many surgeons have experimented with more thinly cut DSAEK grafts. The average DSAEK graft is approximately 130µm to 150µm thick. Ultrathin DSAEK grafts are thinner than 130µm but thicker than 50µm, while nanothin grafts are 50µm.37,38 Some research suggests DMEK patients still may achieve, on average, superior visual function compared with ultrathin DSAEK patients.39
 |
| Fig. 6. This DALK had an incomplete dissection to Descemet’s membrane in surgery that resulted in a hazy interface. The patient later went on to require a PK to remove this haze. Click image to enlarge. |
Takeaways
The outcome and use of corneal transplants are governed by the immunology and longevity of the transplanted cells, the optical and immunologic influence of any sutures that are used and the surgical complexity. Challenges facing all existing and future transplant options will be governed by these same principles.
Dr. Bronner practices at Pacific Cataract and Laser in Boise, ID. He has no financial interests to disclose.
1. Niederkorn JY. Mechanisms of corneal graft rejection: the sixth annual Thygeson Lecture presented at the Ocular Microbiology and Immunology Group meeting. October 21, 2000. Cornea. 2001;20:675-9. 2. Kim SW, Lee HY, Kim TI, et al. The survival of donor-derived cells in a successfully grafted corneal button 10 years after penetrating keratoplasty for lattice dystrophy. Ophthalmologica. 2009;223:396-400. 3. Lagali N, Stenevi U, Claesson M, et al. Survival of donor-derived cells in human corneal transplants. Invest Ophthalmol Vis Sci. 2009;50:2673-8. 4. Patel SV, Hodge DO, Bourne WM. Corneal endothelium and postoperative outcomes 15 years after penetrating keratoplasty. Trans Am Ophthalmol Soc.2004;102:57-66. 5. Cheng YY, Visser N, Schouten JS, et al. Endothelial cell loss and visual outcome of deep anterior lamellar keratoplasty vs. penetrating keratoplasty: a randomized multicenter clinical trial. Ophthalmology 2011;118:302-9. 6. Bourne WM, Nelson LR, Hodge DO. Central corneal endothelial changes over a ten-year period. Invest Ophthalmol Vis Sci. 1997;38:779-82. 7. Bachman B, Taylor RS, Cusiefen C. Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty; an evidence-based meta-analysis. Ophthalmology. 2010;117: 1300-5. 8. Panda A, Vanathi M, Kumar A, et al. Corneal graft rejection. Surv Ophthalmol. 2007;52:375-96. 9. Alldredge OC, Krachmer JH. Clinical types of corneal transplant rejection. Arch Ophthalmol. 1981;99:599-604. 10. Sinha R, Jhanji V, Verma K, et al. Efficacy of topical cyclosporine A 2% in prevention of graft rejection in high-risk keratoplasty: a randomized controlled trial. Graefes Arch Clin Exp Ophthalmol. 2010;248:1167-72. 11. Javadi MA, Feizi S, Karbasian A, et al. Efficacy of topical ciclosporin A for treatment and prevention of graft rejection in corneal grafts with previous rejection episodes. Br J Ophthalmol. 2010;94:1464-7. 12. Magalhaes OA, Marinho DR, Kwitko S. Topical 0.03% tacrolimus preventing rejection in high-risk corneal transplantation: a cohort study. Br J Ophthalmol. 2013;97:1395-8. 13. Patel SV. Graft survival and endothelial outcomes in the new era of endothelial keratoplasty. Exp Eye Res. 2012;95:40-7. 14. Bourne WM. Cellular changes in transplanted human corneas. Cornea. 2001;20:560-9. 15. Lim L, Pesudovs K, Coster DJ. Penetrating keratoplasty for keratoconus: visual outcome and success. Ophthalmology. 2001;107:1125-31. 16. Riddle HK, Parker S, Price FW. Management of postkeratoplasty astigmatism. Curr Opin Ophthalmol. 1998;9:15-28. 17. Hopkinson C, Tole D, Chow SP, et al. Stability of visual outcome after corneal transplantation. ARVO 2016. Seattle, WA; May 1-5, 2016. 18. Cursiefen C, Cao J, Chen L, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci. 2004;45:2666-73. 19. Cursiefen C, Chen L, Dana MR, et al. Corneal Lymphangiogenesis: evidence mechanisms and implications for corneal transplant immunology. Cornea. 2003;22:273-81. 20. Williams KA, Coster DJ. The immunobiology of corneal transplantation. Transplantation. 2007;84:806-13. 21. Dana MR, Qian Y, Hamrah P. Twenty-five year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea 2000;19:625-43. 22. Maguire MG, Stark WJ, Gottsch JD, et al. Risk Factors for corneal graft failure and rejection in the collaborative corneal transplantation studies. CCTSRG. Ophthalmology. 1994;101:1536-47. 23. Eye Bank Association of America. 2019 Eye Banking Statistical Report. 2020 www.restoresight.org. 24. Chan C, et al. Penetrating keratoplasty: the fundamentals. In: Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea. 4th ed. St. Louis: Elsevier; 2017:1265-82. 25. Anshu A, Price MO, Tan DTH, et al. Endothelial keratoplasty: a revolution in evolution. Surv Ophthalmol. 2012;57: 236-52. 26. Tan D, Ang M. Surgical technique for DSEK. In: Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea. 4th ed. St. Louis: Elsevier; 2017:1438-48. 27. Anshu A, Price MO, Price F. Risk of corneal transplant rejection significantly reduced with Descemet’s membrane endothelial keratoplasty. Ophthalmology. 2012;119:536-40. 28. Cheng YY, Visser N, Schouten JS, et al. Endothelial cell loss and visual outcome of deep anterior lamellar keratoplasty vs. penetrating keratoplasty: a randomized multicenter clinical trial. Ophthalmology 2011;118:302-9. 29. Wu SQ, Zhou P, Zhang B, et al. Long-term comparison of full-bed deep lamellar keratoplasty with penetrating keratoplasty in treating corneal leucoma caused by herpes simplex keratitis. Am J Ophthalmol. 2012;153:291-9. 30. Reinhart WJ, Musch DC, Jacobs DS, et al. Deep anterior lamellar keratoplasty as an alternative to penetrating keratoplasty. A report by the American Academy of Ophthalmology. Ophthalmology. 2011;118:209-18. 31. Rafat M, Jabbarvand M, Sharma N, et al. Bioengineered corneal tissue for minimally invasive vision restoration in advanced keratoconus in two clinical cohorts. Nat Biotechnol. 2022; https://doi.org/10.1038/s41587-022-01408-w. 32. Holland G, Pandit A, Sanchez-Abella L, et al. Artificial cornea: past, current, and future directions. Front Med. 2021;8:770780. 33. Franceschino A, Dutheil F, Pereira B, et al. Descemetorhexis without endothelial keratoplasty in Fuchs endothelial corneal dystrophy: a systemic review and meta-analysis. Cornea. 2022;41:815-25. 34. Moloney G, Petsoglou C, Ball M, et al. Descemetorhexis without grafting for Fuchs endothelial dystrophy – supplementation with topical ripasudil. Cornea. 2017;36:642-8. 35. Gupta PK, Kim MJ, Kim T. Developmental corneal anomalies of size and shape. In: Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea. 4th ed. St. Louis: Elsevier; 2017:599-609. 36. Tong CM, Dijk K, Melles GRJ. Update on Bowman layer transplantation. Curr Opin Ophthalmol. 2019;30:249-55. 37. Busin M, Scorcia V. Ultrathin DSEK. In: Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea. 4th ed. St. Louis: Elsevier; 2017:1461-67. 38. Kurji KH, Cheung AY, Eslani M, et al. Comparison of visual acuity outcomes between nanothin Descemet stripping automated endothelial keratoplasty and Descemet membrane endothelial keratoplasty. Cornea. 2018;37:1226-31. 39. Chamberlain W, Lin CC, Austin A, et al. Descemet endothelial thickness comparison trial: a randomized trial comparing ultrathin Descemet stripping automated endothelial keratoplasty with Descemet membrane endothelial keratoplasty. Ophthalmology. 2018;126:19-26. |


