As the emphasis in healthcare continues to shift toward prevention and early detection of disease, eye care practitioners have seen technological innovations emerge that are designed to streamline diagnosis and patient management. Real-time data collection during the patient encounter, called point-of-care testing (POCT), is performed near or at the site of a patient visit, with results available quickly enough to influence the course of care right at the outset, allowing for faster clinical decision-making. Empowering providers to make decisions chairside may have a huge impact in quality of care while also reducing costs to patients and payers.
Ocular surface disease (OSD) is one area of eye care that has recently seen a boom in point-of-care diagnostics. Noting the comorbidity associated with OSD, the prevalence data is extremely telling: an estimated 20.7 million Americans exhibit clinically significant signs or symptoms of dry eye, with more recent studies on the prevalence of meibomian gland dysfunction (MGD) suggesting this number may in fact be significantly higher.1
When one accounts for patients with other forms of ocular surface compromise, the need for greater vigilance for OSD grows even more. Fifty million Americans have allergies, with three million of those individuals suffering from seasonal forms.2 An estimated 38 to 40 million contact lens wearers exist in the United States, while 700,000 individuals undergo LASIK annually across the country.
Data from a 2015 report notes that 68% of US adults own a smartphone and 45% also own a tablet computer; both of these devices have been shown to decrease blink rate and increase the risk of MGD and evaporative dry eye. Symptoms consistent with dry eye are increasing among individuals under the age of 40, who spend on average about two hours per day more on their digital devices than their over-40 counterparts.3 Additionally, Sjögren’s syndrome, a systemic autoimmune disease in which immune cells attach and destroy host exocrine glands that produce tears and saliva, affects about four million people in the United States.
Given this huge potential population base, it can be said that the eye care profession has seen remarkable advancements in POCT over the last decade that have given practitioners the ability to better differentially diagnose underlying causes of ocular surface disease, and therefore make treatments more targeted, timely and cost effective. In this article, several experts on dry eye and ocular surface disease will discuss point-of-care testing and its impact on their dry eye practices.
My Favorite Things
 |
| Moderate to strong positive MMP-9 results on a patient with dry eye disease and conjunctivochalasis. |
Our panelists begin by sharing their impressions of the POC tests for dry eye they find most essential.
Richard Mangan, OD, Moderator. We have come a long way in recent years in our understanding of the underlying cause of both aqueous-deficient and evaporative dry eye, as well as the allergic, infectious and environmental factors that can influence the ocular surface in other ways. The addition of certain point-of-care tests to the subspecialty of ocular surface disease has only broadened our understanding of this multifactorial disease; however, before we get into how POCT has personally impacted your practice, I would like to ask each of you the following question: of the available point-of-care diagnostic tests used in ocular surface disease, if you could only pick one to use in your clinical practice, which one would it be and why?
Scott Schachter, OD. InflammaDry (Rapid Pathogen Screening) is the POC test I rely on and use the most, for several reasons. It measures matrix metalloproteinase 9, or MMP-9, a nonspecific biomarker for inflammation. Studies have shown that desiccating stress, which most all of our patients are exposed to through device use, causes corneal epithelial cells to produce MMP-9.4 Other studies demonstrate a strong correlation between certain levels of MMP-9 and dry eye symptoms, low contrast visual acuity and tear film break-up time.5 MMP-9 is a gelatinase, which weakens epithelial barrier function. If it is present in large enough quantities, the patient essentially has a toxic tear film.
We screen all our patients using the SPEED questionnaire (Standard Patient Evaluation of Eye Dryness), and if they score seven or higher, the staff at the clinic automatically knows to perform this test during pretesting. They write the patient’s initials on the test, and the time that the sample was taken. The test is quick and easy to administer, is reimbursed by most insurers and has results ready in 10 minutes. If positive, I know to start anti-inflammatory therapy; however, if negative, inflammation related to elevated MMP-9 cannot necessarily be ruled out, as false negatives can still occur with this test. If the clinical signs and symptoms suggest a high probability for inflammation, having the patient back another day to repeat the test may be a good idea.
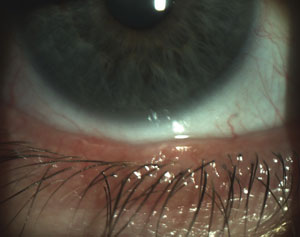 | 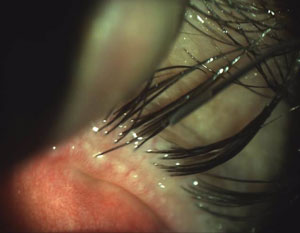 |
| Scalloped lid margins are indicative of the inflammation patterns associated with MGD onset. | Obstructed meibomian glands with telangiectasia also indicate the possible presence of MGD. |
| Photos: Chandra Mickles, OD | |
Regarding the test itself, sensitivity is estimated at 81% to 85% and specificity is 94% to 98%.13 According to another study, other common tests exhibited either poorer sensitivity (i.e., corneal staining at 54%, conjunctival staining at 60% and meibomian gland grading at 61%) or poorer specificity (tear film break-up time at 45% and Schirmer test at 51%) in contrast to this test.6 It’s a single-use test that requires no capital outlay and no volume-based contract, and requires that the tear sample be absorbed from the palpebral conjunctiva, rather than scraped, which can cause additional inflammation or irritation. Practitioners should also keep in mind that test results can take longer than 10 minutes to achieve. Typically, a negative test should be checked again right before the patient leaves the office, just in case it simply has not developed yet.
Scott Hauswirth, OD. I agree with Dr. Schachter. Given the choice of only one, InflammaDry is also my selection for the reasons he has already listed. Though it does not give a complete picture of the inflammatory process, it is probably the most useful tool regarding the guidance of treatment and management. Where I think it has been most helpful is in identification of our more chronic dry eye patients’ MMP-9 levels, which—when taken as an indicator for the overall level of inflammation present on the ocular surface—is often much more elevated than what we may initially think. This helps practitioners determine the success of both the acute control of inflammation with an initial anti-inflammatory pulse and our ongoing maintenance and protective treatments, and allows us the ability to tailor our treatments to gain control of the disease more efficiently and effectively.
Whitney Hauser, OD. Obtaining an osmolarity measurement is my go-to choice. It’s been well-established that tear film osmolarity is accurate for both the diagnosis and classification of dry eye disease; however, research has also shown that its utility goes beyond a conventional dry eye POC diagnostic.6 In contact lens wear, osmolarity can be influenced by the lenses themselves as well as the specific type of solution used.7 Interestingly, scleral contact lenses, often used to treat some of the most symptomatic dry eye patents, can also cause a statistically significant increase in osmolarity after one month of wear; in a study, tear osmolarity measurements were found to increase at a statistically significant level after one month of scleral contact lens wear.8 Reduced basal tear production and the disruption of the tear film layer is believed to contribute to this increase in tear osmolarity.
From a practice management perspective, osmolarity is billable (CPT 83861, Microfluidic analysis utilizing an integrated collection and analysis device) and data can be gathered by a trained staff member.
Dr. Mangan. I have quite a bit of experience using both the RPS InflammaDry test and the Tearlab osmolarity system. Both have been true POC tests for me in that results have influenced my decision on whether to treat using anti-inflammatories. While a negative test does not rule out the presence of inflammatory dry eye disease, it does make me pause and explore other potential causes for their symptoms.
In accordance with what Dr. Hauser offered, there is compelling data and a number of clinical studies to support the idea of using a product like Tearlab, not only in our dry eye clinics, but during the course of a routine examination with our contact lens wearers, as well as our cataract and refractive patients.9-11 A study presented by R. Doyle Stulting, MD, at the 2014 ESCRS meeting in London suggests a strong correlation between a hyperosmolar tear film and the risk of refractive surprise due to more variable keratometry readings. According to the published research, 17% of hyperosmolar eyes exhibited more than 1D of change in K cylinder between the first and second preoperative visits.10 This is just one more study to support the idea that surface optimization is important with respect to surgical outcomes and postoperative satisfaction scores.
One condition that we know can lead to severe inflammatory dry eye is Sjögren’s syndrome (SS). According to the DEWS report, SS is a chronic systemic inflammatory disorder characterized by lymphocytic infiltration of exocrine glands. This often results in xerophthalmia (dry eyes), and xerostomia (dry mouth), and parotid gland enlargement. Primary Sjögren’s syndrome occurs in the absence of another underlying rheumatic disorder, whereas secondary Sjögren’s syndrome is associated with another underlying rheumatic disease, such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) or scleroderma.
Traditionally, clinicians adhere to one of a number of classification systems in attempting to diagnose SS in the absence of biopsy results (see “Classification Systems for Sjögren’s Syndrome,” p. 37). Serology is ordered looking for inflammatory markers like anti-nuclear antibody (ANA) with instructions for the lab to run an SS-A (Ro) and SS-B (La) if the patient’s ANA was elevated. Rheumatoid factor levels should also be measured. Note, research indicates that ANA is expressed in only 60% of patients with Sjögren’s syndrome, and the disease process can be active for years before traditional biomarkers like Ro and La become evident or positive.1,12
Dr. Hauswirth. The Sjö test (Bausch + Lomb) has been an extremely useful addition to our tool chest. First, the potential implications of hastening the diagnosis and early intervention of a life-altering disease are tremendous. Use of this test to identify patients with Sjögren’s syndrome earlier on in their disease state and help them begin to establish relationships with rheumatology is critical in staying ahead of the condition’s progression. In my own practice, we have been able to identify many individuals as early-stage Sjögren’s patients who are now collaborating with rheumatology in their care.
This has potentially life-altering implications, as the 15-year risk for a Sjögren’s patient to develop non-Hodgkin’s B-cell lymphoma is nearly 10%, and the site of lymphoma is often the marginal zone histological type, and aggregate to the mucosa-associated lymphoid tissue where Sjögren’s inflammation is most active.13 This in addition to the extraglandular involvement of organ systems found in 75% of primary SS patients.14 I think this justifies our efforts to identify the disease as early as possible, and allows our colleagues in rheumatology and other subspecialties to collaborate to our patient’s benefit.
| Table 1. Biomarkers Measured in the Sjö Test Diagnostic Panel | ||
| Biomarker | Diagnostic Characteristics | |
| Novel, proprietary | Salivary protein-1 (SP-1, IgA, IgC, IgM) | Provides high specificity and sensitivity for early Sjögren’s syndrome. |
| Carbonic anhydrase (CA-6, IgA, IgC, IgM) | Offers additional sensitivity for an early diagnosis. | |
| Parotic secretory protein (PSP, IgA, IgC, IgM) | Expressed early in disease course. | |
| Traditional | SS-A (Ro) | Expressed in about 70% of patients; typically appears later than the novel biomarkers. |
| SS-B (La) | Less frequently expressed than Ro; typically appears later than novel biomarkers. | |
| Antinuclear antibody (ANA) by HEp-2 | Expressed in about 60% of Sjögren’s syndrome patients. | |
| Rheumatoid factor (RF) levels (IgA, IgC, IgM) | Found in many rheumatic conditions—not unique to Sjögren’s syndrome. | |
| Courtesy Bausch + Lomb | ||
The following case helps to demonstrate the value of incorporating Sjö into an eye care practice:
A 36-year-old Caucasian female presented via referral for symptoms of dry eye following laser vision correction more than two years ago. She reported a positive family history of rheumatoid arthritis. After attempting compliance with treatments including warm compresses, artificial tears PRN, Restasis (cyclosporine 0.05%, Allergan) BID and Lastacaft (alcaftadine 0.25%, Allergan) QD OU, she presented with the feeling that her eyes are worsening, with significant burning, pain and visual fluctuation. Additionally, though the patient reports no joint or back pain, she does note that her mouth has been drier than normal during the last year.
Entrance acuities of 20/40 and 20/50, respectively, were recorded, while a slit lamp exam revealed 3+ PEK with filaments, scant tear film and rapid TBUT OU. Treatment was modified to include insertion of Freeman-style punctal plugs in the lower lids OU and use of preservative-free artificial tears QID OU, Restasis BID OU and FML (fluorometholone) QID OU. Additionally, a Sjö test was ordered and the patient was directed to return in one month for follow-up.
Upon return, the patient reported that her symptoms were still present, yet improved. BCVA was 20/30 and 20/50, respectively. Slit lamp exam demonstrated a 2+ central PEK OD, 3+ OS but no filaments. Treatment was once again modified to include same-day punctal occlusion of the upper lids and she was switched from FML to Lotemax gel (loteprednol etabonate 0.5%, Bausch + Lomb) QID OU. Sjö was performed and it was revealed that both traditional and novel biomarkers were abnormal, suggesting this patient had Sjögren’s syndrome. A rheumatology consult was scheduled, and the patient was formally diagnosed with primary Sjögren’s based on the reported symptoms, physical signs and Sjö test findings.
She was given patient education materials on SS and advised to discuss resuming Restasis, as it could preserve remaining lacrimal glands from further destruction. A recommendation of Biotene products for the dryness of mouth was made, as the symptom was not severe enough to warrant treatment with cholinergic drugs like Evoxac (cevimeline hydrochloride, Daiichi-Sankyo). She was informed that patients with Sjögren’s are at high risk of having lymphoma and asked to undergo labs at the time and six months later. She was advised to inform her doctor is she develops any fever, chills or night sweats, or if she notices any enlarged lymph nodes. She was also informed that patients with +SSA/SSB have a 1% chance of having a child with congenital heart block, so cardiac monitoring with weekly fetal echocardiogram is warranted during any future pregnancy.
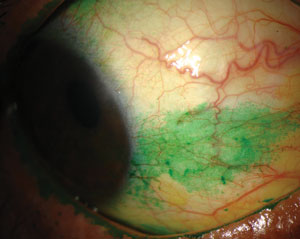 |
 |
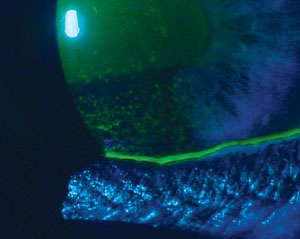 |
| Signs and symptoms for dry eye diagnosis. Top to bottom: (a) conjunctival staining with lissamine green in a dry eye patient; (b) staining with lissamine green on the inferior cornea; and (c) presence of minimal tear meniscus indicates a reduction in tear film quantity. Photos (from top to bottom): Michelle Hessen, OD, Ben Gaddie, OD, Paul M. Karpecki, OD. |
Dr. Hauser. Sjögren’s is a frequently misdiagnosed and underdiagnosed disease. Just over one million people in the United States are diagnosed with it, and it’s estimated that as many as four million individuals are suffering from it.15 Considering that ocular surface complaints are extremely common and may be some of the earliest and most compelling signs and symptoms to present, eye care providers have a distinct opportunity to act as the gatekeepers for this elusive autoimmune disease. As such, the Sjö test acts as an ideal screener. As Dr. Hauswirth said, working closely with rheumatology is critical.
The American-European Consensus Criteria for Sjögren’s Syndrome found only one sign and one symptom commonly associated with dry eye disease in combination with oral signs and symptoms warrants additional investigation. As such, histological and antibody testing for anti-SSA (Ro) anti-SSB (La) or both are essential for completing the diagnosis.16 While convention relies on the traditional biomarkers, these often fall short in early diagnosis, as Dr. Mangan recognized. The Sjö test includes three traditional biomarkers and four novel ones, which may be expressed earlier in the disease process (Table 1).17
Looking at the Glands
Moving on to pathophysiology evaluation, panelists consider the role of the relevant ocular anatomy and how to evaluate it noninvasively.
Dr. Mangan. I would like to now turn our attention to meibomian gland dysfunction (MGD). According to the executive summary from the International Workshop on Meibomian Gland Dysfunction, MGD is a chronic, diffuse abnormality of the meibomian glands that is commonly characterized by terminal duct obstruction and/or qualitative/quantitative changes in the glandular secretion. It may result in alteration of the tear film, symptoms of eye irritation, clinically apparent inflammation and ocular surface disease. The MGD workshop additionally proposed that MGD may well be the leading cause of dry eye throughout the world.18
Like aqueous-deficient dry eye, MGD is a multifactorial disease. To date, epidemiology and prevalence data is inconsistent, with possible risk factors still being researched. With that being said, we know that MGD can affect a wide range of age groups. We are seeing more reports of how excessive use of digital devices is adversely affecting blink rate and lid mechanics, secondarily causing meibomian gland dropout and evaporative dry eye symptoms at a much earlier age.
Given all of this information, how has meibography influenced your approach to managing MGD?
Dr. Schachter. Meibography has had a significant impact on how we diagnose and treat MGD. As we have looked more, we have found more. This has led us to image younger and younger patients. Teens and young adults spend the majority of their day looking at electronic devices, and most of us believe that this is leading to meibomian gland atrophy. In fact, in my practice we are approaching a “screen everyone” protocol for the lower glands only, and we can now intervene sooner as we find atrophy sooner. The new Lipiscan (TearScience) should allow us to image quickly.
Imaging technology is also an excellent patient education tool, as we can now show patients their ocular anatomy and also any meibomian gland dropout. This helps to drive patient compliance with our recommendations, which typically include some combination of blink exercises, warm compresses, debriding, Lipiflow, nutraceuticals, artificial tears, lid hygiene and Blephex.
Dr. Hauser. Considering that 86% of dry eye disease is evaporative in nature, evaluation of the glands in an essential diagnostic procedure.19 While I enjoy the use of both the LipiView and Oculus Keratograph technology in my office, performing a simple transillumination of the meibomian glands offers a more basic view as well. Determining the structure, number and level of atrophy is valuable in creating a treatment plan and setting the patient’s expectations. Many eye care providers recommend the use of warm compresses without ever visualizing the glands. However, a patient with virtually no glands will be disappointed by their failure to significantly improve from warm compresses. On the other hand, however, while the majority of patients have evaporative dry eye disease, a patient with ideal meibography and lipid layer thickness may suggest another etiology.
Dr. Hauswirth. Meibography has been an incredible asset, though we are still in the early stages of knowing exactly what the causative factors are regarding several aspects to alterations in meibomian gland morphology. Still, it is an incredibly helpful tool from a prognostic and diagnostic standpoint—being able to capture images and display them for patients to illustrate their pathophysiology is a powerful thing. No other test we perform commands a patient’s attention as well as an image of their own glands. In our practice, we have the Oculus Keratograph, Lipiview II and LipiScan. I think all are excellent in performing meibography, although I find the images in the TearScience technology platforms to be sharper.
Dr. Mangan. I currently use the Oculus Keratograph in one of my primary offices, and I find it very useful in both diagnosis of MGD and in determining the location and severity of gland atrophy in my evaporative dry eye patients. We now know that the location of gland atrophy is very important due to the work of Blackie and Korb, as well as Pflugfelder et al. and other teams.20,21 The glands that offer the greatest amount of meibum secretion are the nasal ones, which are responsible for 70% of total secretory volume. Thus, it stands to reason that if there is significant gland fallout nasally, patients are more apt to have more significant disease.
I agree with Dr. Hauser that lid transillumination works well when advanced meibography systems are not available. Transillumination of the lids is part of my general work-up regardless. I find that I can predict the Keratograph results through transillumination. Still, Keratograph images do allow me to hammer the point home when educating my patients about their condition.
Future Advances
In this rapidly evolving category of eye care, where might the technology be heading?
| Classification Systems for Sjögren’s Syndrome | |
| American-European Consensus Group.These criteria allow for a diagnosis in patients without sicca symptoms or who have not undergone biopsy.2,22 Diagnosis of primary Sjögren’s requires at least one of the four criteria below to be present and either criterion #5 or #6 must also be included. Sjögren’s can be diagnosed in patients who have no sicca symptoms if three of the four objective criteria are fulfilled. These criteria are: | American College of Rheumatology. The ACR classification criteria were developed to improve the specificity of the criteria used for entry into clinical trials, especially following the emergence of biologic agents as potential treatments for Sjögren’s syndrome and their associated comorbidities. This high specificity makes the ACR criteria more suitable for application in situations in which misclassification can pose a risk to the patient’s health. These guidelines were accepted by the ACR as a provisional criteria set in 2012.24 |
Dr. Mangan. Next question: Where would you most like to see POC testing improve or advance in the field of ocular surface disease?
Dr. Schachter. Though MMP-9 is very useful when looking for inflammation, there are also other mediators and biomarkers. While a positive result means inflammation of some kind is likely present, a negative result doesn’t necessarily rule it out, as MMP-9 is a non-specific biomarker. Interestingly, it would be helpful if we could take a tear sample and test for other inflammatory biomarkers, such as IFN-gamma—however, we can’t at this time.
Osmolarity is also meaningful and can help direct treatment, allowing us to monitor for efficacy. The problem is, however, that osmolarity can vary over the surface of the eye.
Also, InflammaDry and TearLab require some level of skill in sample collection. InflammaDry test results can be difficult to interpret when MMP-9 levels are just above the cutoff for a positive test, 40ng/ml. Sample collection is also difficult on patients who are very dry. Regardless, as new tests are developed, high sensitivity and specificity are critical, in addition to positive and negative predictive values.
Dr. Hauswirth. I believe the direction we’re heading—toward broader spectrum panels of inflammatory markers, as well as improved imaging of the glands and other important ocular surface structures down to the cellular level—can help us become more accurate in setting treatment strategies. We are in what I call the Model T phase of diagnostic technology for dry eye: benefiting greatly from its existence while recognizing limitations this early in its development. However, understanding those limitations allows us to remodel and refine our tools, making them increasingly helpful and clinically relevant.
Another tool that I believe can be quite helpful is the confocal microscope, which allows us to image through corneal and conjunctival tissue to view active dendritic cells in the cornea, activated keratocytes and corneal nerve morphology, which gives us an image on the actual mechanics of disease in vivo. This is not necessarily practical to implement in a standard eye care practice yet, but the technology is improving, and it has great potential for diagnosis and management.
Dr. Hauser. The greatest potential in POC testing, in my opinion, is the use of multiple tests in concert. We have several useful, but often underused, tests available today. Ideally in the future, practitioners will be able to perform multiple objective tests to better address the multifactorial nature of the condition and to objectively monitor improvement or progression.
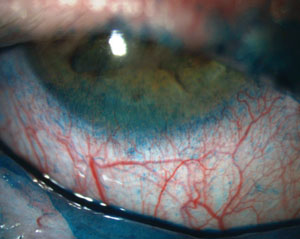
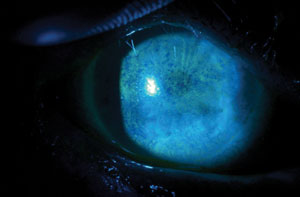 |
| Fluorescein staining of a patient with severe punctate epithelial keratopathy secondary to dry eye that is associated with Sjögren’s syndrome and exposure keratopathy. Photos: Ben Gaddie, OD |
Dr. Mangan. Thank you. I think we can all agree that each POCT mentioned in this article brings some amount of value and information to the overall picture of patient ocular surface health. With that in mind, however, each of these tests also has certain limitations regarding the information it can reveal.
I personally have had patients who were diagnosed with primary Sjögren’s display a normal osmolarity reading in both eyes. Another patient tested negative in the InflammaDry test during one visit but positive a month later, without any change in how they were being treated in between the two visits. As such, I definitely agree with Dr. Hauswirth that we are only in the beginning stages of where POCT may eventually progress to. However, the POCT tests discussed here may provide some information that can influence chairside treatment decisions in today’s practices.
In closing, I would like to thank the panel for sharing their thoughts regarding how they use point-of-care technology in their dry eye clinics. I, for one, am encouraged by the industry’s continuing interest in this area and am excited to see it progress. I hope that eye care practitioners across the country understand not only the need but also the opportunity that is available to them to become more focused in the diagnosis and management of ocular surface disease.
1. Harmon D. Market Scope, LLC. 2008 Comprehensive Report on the Global Dry Eye Market. Available at: http://dev.market-scope.com/market_reports/2008/07/2008-comprehensive-report-on-t.html. Accessed July 7, 2009.
2. American Academy of Allergy, Asthma & Immunology (AAAAI). Diseases 101: Asthma and Allergy Statistics. Available at: www.aaaai.org/patients/gallery/prevention.asp?item=1a. Accessed July 7, 2009.
3. Harthan J, O’Dell L, Kwan JT, et al. Dry Eye Symptoms and Visual Function with Digital Device Use. ARVO 2016; abstract #2843-A0052.
4. Corrales RM, Stern ME, De Paiva CS, et al. Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Invest Ophthalmol Vis Sci. 2006 Aug;47(8):3293–302.
5. Kaufman HE. The practical detection of mmp-9 diagnoses ocular surface disease and may help prevent its complications. Cornea. 2013 Feb;32(2):211-6.
6. Lemp MA, Bron AJ, Baudouin C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011 May;151(5):792-8.
7. Karkkainen TR, Smith MK, Wood JR. The effect contact lens solution osmolarity has on tear film toxicity. Invest Ophthalmol Vis Sci. 2002 Dec;32(13):3090.
8. Lau J, Gao C, Chaglasian E. The effect of scleral lens wear on tear osmolarity. Poster. American Academy of Optometry 2015.
9. Iskeleli G, Karakoc Y, Aydin O, Yetik H, Uslu H, Kizikaya M. Comparison of tear-film osmolarity in different types of contact lenses. CLAO J. 2002 Oct;28(4):174-6.
10. Epitropoulos AT, Matossian C, Berdy GJ, Malhotra RP, Potvin R. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J Cataract Refract Surg. 2015;41:1672-7.
11. Giancarlo M. Modification of the tear film osmolarity with the use of contact lenses in omafilcon A and methafilcon A materials. BCLA poster presentation. 2010.
12. Clinical Laboratory International Online. Sjögren’s syndrome: an under-diagnosed disorder. Available at www.cli-online.com/fileadmin/pdf/pdf_general/sj%F6gren’s-syndrome-an-underdiagnosed-disorder.pdf. Accessed July 7, 2016.
13. Solans-Laque R, Lopez-Hernandez A, Bosch-Gill JA, et al. Risk, predictors, and clinical characteristics of lymphoma development in primary Sjogren’s syndrome. Semin Arthritis Rheum. 2011;41(3):415-23.
14. Baldini C, Pepe P, Quartuccio L, et al. Primary Sjogren’s syndrome as a multi-organ disease: impact of the serological profile on the clinical presentation of the disease in a large cohort of Italian patients. Rheumatology. 2014;53(5):839-44.
15. Dysautonomia International. Underlying Causes of Dysautonomia. Available at: www.dysautonomiainternational.org/page.php?ID=150. Accessed July 7, 2016.
16. John Hopkins Medicine. American-European Consensus Criteria for Sjögren’s Syndrome. Available at www.hopkinssjogrens.org/disease-information/diagnosis-sjogrens-syndrome/americaneuropean-consensus-criteria-sjgrens-syndrome/. Accessed July 7, 2016.
17. Kent C. Using today’s dry eye diagnostic tools. Rev Ophthalmol. 2015 Oct; 22(10):22-34.
18. Nichols KK, Foulks GN, Bron AJ, et al. The International Workshop on Meibomian Gland Dysfunction: Executive Summary. Invest Ophthalmol Vis Sci. 2011 Mar;52(4):1922-9.
19. Lemp MA, Crews LA, Bron AJ. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012 May;31(5):472-8.
20. Blackie CA, Korb DR, Knop E, et al. Nonobvious obstructive meibomian gland dysfunction. Cornea. 2010 Dec;29(12):133-45.
21. Pflugfelder SC, Tseng S, Sanabria O, et al. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea. 1998 Jan;17(1):38–56.
22. Kassan SS, Moutsopoulos HM. Clinical manifestations and early diagnosis of Sjögren syndrome. Arch Intern Med. 2004;164:1275-84.
23. American Academy of Allergy, Asthma & Immunology (AAAAI). Diseases 101: Asthma and Allergy Statistics. Available at www.aaaai.org/patients/gallery/prevention.asp?item=1a. Accessed July 7, 2009.
24. American College of Rheumatology. American College of Rheumatology Classification Criteria for Sjögren’s Syndrome: A Data-Driven, Expert Consensus Approach in the Sjögren’s International Collaborative Clinical Alliance Cohort. Available at www.rheumatology.org/Portals/0/Files/2012%20ACR%20Sjogrens%20Classification%20Criteria%20v.pdf. Accessed July 23, 2016.


