The most noteworthy clinical development of the 21st century is likely the evolution of highly resistant bacteria that are being identified in ocular infections.
Traditionally, the ophthalmic arena has been spared most of the concerns associated with resistance of bacteria and efficacy of therapy due to the simple fact that we have the luxury of applying the therapeutic agent directly to the site. To a large degree, this bypasses the concerns associated with GI absorption and access to remote parts of the body. We are also lucky to have a wide array of potential agents available to meet the specific needs of individual patients.
But all that is changing.
History of Bacterial Infections
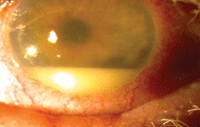
Post-LASIK MRSA.
Over the last seven years we’ve seen a rapid decline in the efficacy of antibiotics and a slowing of the technology pipeline for new drug development. This creates both a current challenge and future concern for all clinicians.1-3 Historically, the most common causes of bacterial infections of the eye have been Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumoniae, Haemophilus influenzae, Pseudomonas aeruginosa, Moraxella, Serratia Marcescens and occasionally, Enterococcus.4 Over the past several decades, these bacteria had shown little change in susceptibility, which has allowed practitioners to manage most topical infections without significant concern. In particular the third- and fourth-generation fluoroquinolones gave us a powerful weapon to address almost any type of infection.
Ophthalmic Infections
A 2008 study by Kara Cavuoto, M.D., looked at bacterial isolates in ophthalmic infections and found an almost three-fold increase in resistance to third-generation fluoroquinolones (ciprofloxacin) relative to S. aureus over a 10-year period (from 1994 to 2003), and an emergence of MRSA from 4.4% to 42.9% of all cultures.5 Additional work by Penny Asbell, M.D., Michael H. Goldstein, M.D., and Fabiana Marangon, M.D., support this concern of increasing resistance in ocular therapy.6-8 In 2009, Marguerite McDonald, M.D., identified a remarkable increase in multidrug resistant isolates to ciprofloxacin in relation to MRSA, which showed that 65% of MRSA isolates and 47% of MRSE isolates also showed ciprofloxacin resistance.9
There has been a number of identified trends in resistance to agents such as azithromycin, gentamicin and tobramycin. Maria Regina Chalita, M.D., showed a decrease of sensitivity for gentimcin and tobramycin from 88% and 95% respectively, to 50% and 80% over a 15-year period.10,11
Bacterial Keratitis
The more significant issue is the growth of resistance in the past decade to organisms involved in sight-threatening infectious keratitis and endophthalmitis. Several studies have identified this pattern of rising resistance related primarily to gram-positive bacteria such as S. aureus. Tristan Bourcier, M.D., Ph.D., conducted a study of 291 patients with bacterial keratitis, noting that that the predominant culture positive bacteria was gram-positive (83%) with a suprisingly low gram-negative (17%).12 This trend is concerning because the antibiotics used to treat microbial keratitis (MK) are still quite effective against gram-negative bacteria (Pseudomonas specifically). However, the loss of efficacy seen in gram-positive coverage has been both rapid and alarming.
MRSA has seen a rapid rise in bacterial isolates, increasing from 29.5% in 2000 to 41.6% in 2005.13 Additionally, the efficacy patterns also underwent significant change: Fourth-generation fluoroquinolones maintained status against methicillin susceptible (MSSA) bacteria, while there was a marked decline in susceptibility against MRSA isolates. This shifting pattern implies significant concerns about the clinical protocols used to manage bacterial keratitis in both contact lens and non-contact lens lesions.
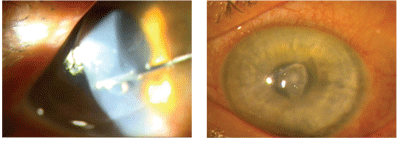
Vancomycin irrigated under the flap in a post-op endophthalmitis eye (left). Four days later (right).
Ocular Infections
In a seminal work by Dr. Asbell and colleagues, the Ocular TRUST (Tracking Resistance in U.S. Today) was developed to address these rapid changes in microbial resistance as they relate to ocular infections.14 The first data report assessed 197 cultures from participating centers and looked at S. aureus, S. pneumoniae and H. influenzae isolates from 2005 to 2006. Antibiotics tested were all the available topical agents used in clinical practice.
The outcomes were both interesting and concerning. Among S. pneemoniae isolates, all were susceptible to the fluoroquinolones except five that showed intermediate resistance to ciprofloxacin. The other agents varied from a low of 18.3% with penicillin to a high of 100% with polymixin B. The H. influenzae isolates showed overall sensitivity to all agents except trimethoprim. The real concern was with S. aureus—agents that showed good efficacy to MSSA, but demonstrated high-level resistance to MRSA. Fourth-generation fluoroquinolones such as moxifloxacin, gatifloxacin and levofloxacin demonstrated 15% sensitivity and over 80% resistance to MRSA isolates.14
A European study also demonstrated the declining efficacy of the fluoroquinolones against MRSA in a review of 582 isolates.15 This has occurred despite initial marketing that indicated that it was nearly impossible for bacteria to evolve resistance because it would require a simultaneous topoisomerase and DNA gyrase mutation. The clinical implications of this information are significant: The agents that have been the backbone of therapy for the last decade are exhibiting an Achilles’ heel that makes treating microbial keratitis a moving target.
Post-Surgery
Another area of concern is the changing pattern of microbial isolates following both cataract and refractive surgery. Eric Donnenfeld, M.D., and colleagues presented data at the 2009 American Society of Cataract and Refractive Surgery meeting showing that 77% of positive lid or conjunctival cultures were either S. epidermidis or S. aureus.16 They also noted that the incidence of colonization of MRSA/MRSE was 33% of all patients assessed, which increased with age to 50% at 80 years old. They identified that colonization of the ocular surface is more likely in health care workers (1.25), age (1.27) and glaucoma patients (1.44).16
Darlene Miller, D.H.Sc., M.P.H., and colleagues showed similar patterns of microbialization in the surgical population, and also found that 65% of patients with ciprofloxacin-resistant organisms demonstrated in vitro cross resistance to moxifloxacin and gatifloxacin.17
Treatment protocols also have begun to change as a result of increasing resistance. The European endophthalmitis trials demonstrated a 78% reduction of incidence with an intraoperative injection of cefuroxime in addition to all typical pre- and post-operative regimens.18 LASIK and PRK surgery also have shown significant increases in the rate of MRSA infections with similar patterns of antibiotic sensitivity.19
These resistance changes are consistent with evolving patterns of microbial behavior, both systemic and global.20 There is evidence that the emerging patterns of MRSA resistance are independent of the traditional hospital-based organisms, and instead represent a trans-species infection pattern that is derived from a separate source.
An October 2007 study reviewed the status of MRSA across the United States and found an occurrence rate of 95,000 cases with a mortality rate of 19,000.21 Compare that statistic with 12,500 AIDS-related deaths during the same peroid.21 A subsequent November 2007 study identified the likely source of this outbreak as the use of low-dose fluoroquinolones in the animal husbandry business to promote faster weight gain in farm animals, specifically swine.22 There is also evidence that this same mechanism may be producing a ciprofloxacin-resistant S. aureus. The original MRSA is now referred to as HB-MRSA (hospital-based) and the new strain is called CA-MRSA (community-acquired).
For practitioners, the primary impact of this finding is in the management of patients who present with MK, with or without contact lens wear.
A Treatment Plan
I still recommend therapy be initiated with a fourth-generation fluoroquinolone at the appropriate dose rate (qh to q2h) and the patient be seen daily until therapy has demonstrated improvement. If, however, at any time in the first 48 to 72 hours, the patient demonstrates regression or lack of progression of the MK, it is reasonable to assume a fluoroquinolone failure and to initiate additional therapeutic intervention to address the issue.
The first step is to culture the patient and send out for laboratory analysis; this is best done via blood and chocolate agars, thioglycollate broth and Sabourauds media. The material should also be plated for Gram and Giemsa stains.
The next step after culture and stain is initiation of fortified antibiotics. While drugs like Polytrim are effective against MRSA conjunctivitis, they are not indicated for suspected MRSA/MK unless they are used only until vancomycin can be obtained. I recommend fortifying the vancomycin to 25mg/ml and dosing at qh, alternating at qh with the fluoroquinolone that was initially used until culture results are back.
In some contact lens-wearing patients, it is also appropriate to start additional gram-negative coverage with an agent like tobramycin, fortified to 14mg/ml, on the same alternating dose to address the possibility of Pseudomonas. In most cases, it is reasonable to simultaneously start doxycycline 100mg p.o. b.i.d. to attempt to delimit the collateral damage from collagenase and the recruitment of cytokines and interleukins. The patient must be seen daily until the culture is reported, and then the clinician can make adjustments based on results.
Due to the aggressive nature of the more resistant bacteria, it is common to have significant tissue damage associated with MK, so consider the use of a steroid after the field has been sterilized with several days of treatment. While each case is unique, the role for the anti-inflammatory properties must be considered but clinicians need to be cautious in implementing it.
Future Considerations
Unfortunately, due the aggressive nature of MRSA, the concern regarding the organism mutating against vancomycin is already a reality. Simon R. Bababeygy, M.D., cited two 2009 cases of preseptal cellulites that were vancomycin resistant but responded to rifampin and linezolid therapy.23
The pharmaceutical pipeline to address the rising rate of resistance is not strong at this time. The only new agent recently released is a chloro-fluoroquinolone, Besivance (besifloxacin 0.6%, Bausch + Lomb). It has demonstrated increased sensitivity to MRSA and MRSE in clinical studies in conjunctivitis.24-26 Its increased efficacy may be related to the unique structure of the molecule.27 While there is little data related to its role in MK management, animal model studies of endophthalmitis compare it to the other fourth-generation agents.
The World Health Organization (WHO) has begun the process of creating guidelines for the worldwide use of antibiotics in an effort to bolster current sensitivities and decrease future resistance development. Recently, WHO issued a guideline for the cessation of antibiotic use in animals, with a complete discontinuation to follow within several years. Given the current issue with trans-species MRSA, this will hopefully begin the process of better balancing our use of antibiotics with our needs.
Dr. Thimons graduated from Ohio State University College of Optometry and has served as chief, Optometric Service at the VA Medical Center in Chillicothe, center director and chairman at Omni Eye Services in Fairfax, Va., director at The Glaucoma Institute at SUNY and executive director at TLC Connecticut. In 2002, he founded the Ophthalmic Consultants of Connecticut.
1. Moran GJ, Talan DA. Community-associated methicillin-resistant Staphylococcus aureus: is it in your community and should it change practice? Ann Emerg Med. 2005 Mar;45(3):321-2.
2. Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus in three communities. N Engl J Med. 2005 Apr 7;352(14):1436-44.
3. Chambers HF. Community-associated MRSA—resistance and virulence converge. N Engl J Med. 2005 Apr 7;352(14):1485-7.
4. Cavuoto K, Zutshi D, Karp CL, et al. Update on bacterial conjunctivitis in South Florida. Ophthalmology. 2008 Jan;115(1):51-6.
5. Bartlett, JD, Jaanus S. Clinical Ocular Pharmacology. MO Butterworth; St. Louis, 2008
6. Asbell PA, Colby KA, Deng S, et al. Ocular TRUST: nationwide antimicrobial susceptibility patterns in ocular isolates. Am J Ophthalmol. 2008 Jun;145(6):951-8.
7. Goldstein MH, Kowalski RP, Gordon YJ. Emerging fluoroquinolone resistance in bacterial keratitis: a 5-year review. Ophthalmology. 1999 Jul;106(7):1313-8.
8. Marangon FB, Miller D, Muallem MS, et al. Ciprofloxacin resistance among methicillin-sensitive Staphylococcus aureus isolates from keratitis and conjunctivitis. AM J Ophthalmol. 2004 Mar;137(3):453-8.
9. McDonald MB, Blondeau JM, DeCory HH, et al. Multidrug-resistant strains in clinical trials of besifloxacin in the treatment of bacterial conjunctivitis. Presented at the American Academy of Ophthalmology annual meeting, November 8-11, 2008; Atlanta.
10. OhnsmanC, Ritterband D, OíBrien T, et al. Comparison of azithromycin and moxifloxacin against bacterial isolates causing conjunctivitis. Curr Med Res Opin. 2007 Sep;23(9):2241-9.
11. Chalita MR, Hofling-Lima AL, Paranhos A Jr, et al. Shifting trends in in vitro antibiotic susceptibilities for common ocular isolates during a period of 15 years. AM J Ophthalmol. 2004 Jan;137(1):43-51.
12. Bourcier T, Thomas F, Borderie V, et al. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. BR J Ophthalmol. 2003 Jul;87(7):834-8.
13.Asbell PA, Sahm DF, Shaw M, et al. Increasing prevalence of methicillin resistance in serious ocular infections caused by Staphylococcus aureus in the United States: 2000 to 2005. J Cataract Refract Surg. 2008 May;34(5):814-8.
14.Asbell PA, Sahm DF. Longitudinal nationwide antimicrobial susceptibility surveillance in ocular isolates: results from Ocular TRUST 2. Presented at the American Society of Cataract and Refractive Surgery annual meeting, April 27-May 2, 2007; San Diego, Calif.
15. Morrisey I, Burnett R Viljoen L, Robbins M. Survellience of the susceptibility of ocular bacterial pathogens to the fluoroquinolone gatafloxacin and other antimicrobials in Europe during 2001-02. J Infect. 2004;49(2):109-14.
16. Donnenfeld E, Olson R, Solomon K, et al. National surveillance of methicillin-resistant Staphylococcus from the ocular surface of cataract surgery patients. Poster presented at the American Society of Cataract and Refractive Surgery. 2009.
17. Miller D, Flynn PM, Scott IU, et al. In vitro fluoroquinolone resistance in staphylococcal endophthalmitis isolates. Arch Ophthalmol. 2006 Apr;124(4):479-83.
19. Solomon K, Donnenfeld E, Azar D, et al. Infectious keratitis after laser in situ keratomileusis: Results of an ASCRS survey. J Cataract Refract Surgery. 2003 Oct;29(10):2001-6.
18. Barry P, Seal D, Gerrinby G, et al. ESCRS study of prophylaxis of postoperative endophthalmitis after cataract surgery. J Cataract Refract Surgery. 2006 Mar;32(3):407-10.
20 Croft Ac, D’Antoni AV, Terzulli SL. Update on antibacterial resistance crisis. Med Sci Monit. 2007 Jun;13(6):RA 103-18.
21 JAMA. 2007 Oct.
22. Vectors. University of Ontario. 2007 Nov.
23. Bababeygy S. MRSA: Another Change in Plan. Ophth Plastic Recon Surgery. 2009.
24. Blondeau JM. Fluoroquinolones: mechanism of action, classification, and development of resistance. Surv Ophthalmol. 2004;49(Supp 2):S73-8.
25. Karpecki P, DePaolis M, Hunter JA, et al. Besifloxacin ophthalmic suspension 0.6% in patients with bacterial conjunctivitis: a multicenter prospective, randomized, double-masked, vehicle controlled, 5-day efficacy and safety study. Clin Ther. 2009 Mar;31(3):514-26.
26. Tepedino ME, Heller Wh, Usner DW, et al. Phase III efficacy and safety study of besifloxacin ophthalmic suspension 0.6% in the treatment of bacterial conjunctivitis. Curr Med Res Opin. 2009 May;25(5):1159-69.
27. Ward KW, Lepage JF, Driot JY. Nonclinical pharmacodynamics, phar-macokinetics, and safety of BOL-303224-A, a novel fluoroquinolone antimicrobial agent for topical ophthalmic use. J Ocul Pharmacol Ther. 2007;23(3):243-56.


