-1.jpg) |
| Fig. 1. Post-LASIK placido disc topography of a highly myopic patient with a deeply central oblate pattern without ectasia, central islands or irregular astigmatism. Click images to enlarge. |
Reverse-geometry rigid lenses have been the mainstay of post-surgical lens fitting for years. The modality’s flatter posterior design allows a thinner tear layer and a more aligned fit on both oblate and prolate corneas.
From the first laser in situ keratomileusis (LASIK) performed in 1991 and its subsequent FDA approval in 1995, there have been an estimated 10 million Americans who have had LASIK. One study found that myopia greater than 1.00D typically develops in LASIK patients after 10 years; another reported that over half were under- or over-corrected by at least 1.00D at the 15-year mark.1,2 The number of post-LASIK patients in need of corrective lenses to treat their post-refractive regression will only continue to grow as the number of surgeries increases.
This case report discusses a specialty contact lens fit on a patient with remarkably oblate corneal status post-LASIK, the factors that contribute to post-surgical myopic regression and the pathophysiology of the most common post-LASIK complication: dry eye. If you’ve ever experienced a similar presentation, pay attention to the takeaways offered here so you’ll be better equipped moving forward.
The Case
A 54-year-old Asian male presented with blurry vision and glare at night that had gradually worsened over several years. Ocular history included uneventful bilateral microkeratome-assisted LASIK in 2002 with a high pre-op myopic astigmatic prescription. He was referred for a specialty contact lens fitting due to unstable soft contact lenses fit.
Uncorrected visual acuities were 20/40±2 that pinhole-corrected to 20/20-2 OD and 20/100-1 that pinhole-corrected to 20/40+2 OS. The patient’s manifest refraction was -1.00+0.75x165 OD and -2.00 OS with an add of +1.75 and visual acuity of 20/20 OU. His cycloplegic refraction was -0.75+0.75x165 with visual acuity of 20/20 OD and -1.75 with visual acuity of 20/20-2 OS.
-2.jpg) |
| Fig. 2. (A) Mild ILZ clearance and satisfactory soft lens cushion support nasally (left) and temporally with UltraHealth FC diagnostic lenses. (B) AS-OCT depicts an ideal apical alignment fit with the lowest vault available in the UltraHealth FC diagnostic lens set. (C) The initial diagnostic lens shows apical alignment and tear layer thinning of the ILZ. (D) Nasal ILZ bearing on the corneal epithelium (left) and minimal tear vault on the temporal ILZ post-30 minutes of diagnostic lens settling. Click image to enlarge. |
Intraocular pressures were 16mm Hg OD and 12mm Hg OS. Slit lamp exam revealed well-healed LASIK flaps without striae, stromal haze, intrastromal debris, epithelial ingrowth or surface punctate epithelial keratopathy. The tear meniscus appeared normal in height bilaterally, but the tear breakup time was reduced by approximately six to seven seconds in each eye. Because post-op dry eye is a common refractive surgery complication, we performed phenol red thread testing to measure basal tear production.3,4 The results yielded 30mm OD and 24mm OS, both of which were normal.
The topographical map of the patient’s right eye depicted a central oblate pattern with uniform mid-peripheral steepening. There was no evidence of corneal ectasia or irregular astigmatism. Similarly, the left eye showed an even larger area of central flattening with uniform mid-peripheral steepening that indicated a well-centered flap, well-aligned ablation of the stromal tissue and absence of any central islands (Figure 1).
Due to the highly oblate topographies, we selected the SynergEyes UltraHealth FC hybrid contact lens for the diagnostic fit. As topography revealed deeply flat bilateral keratometric readings, we assumed the patient’s pre-op K readings were also on the flatter end of the spectrum and, thus, chose a lower vault for the initial diagnostic lens.
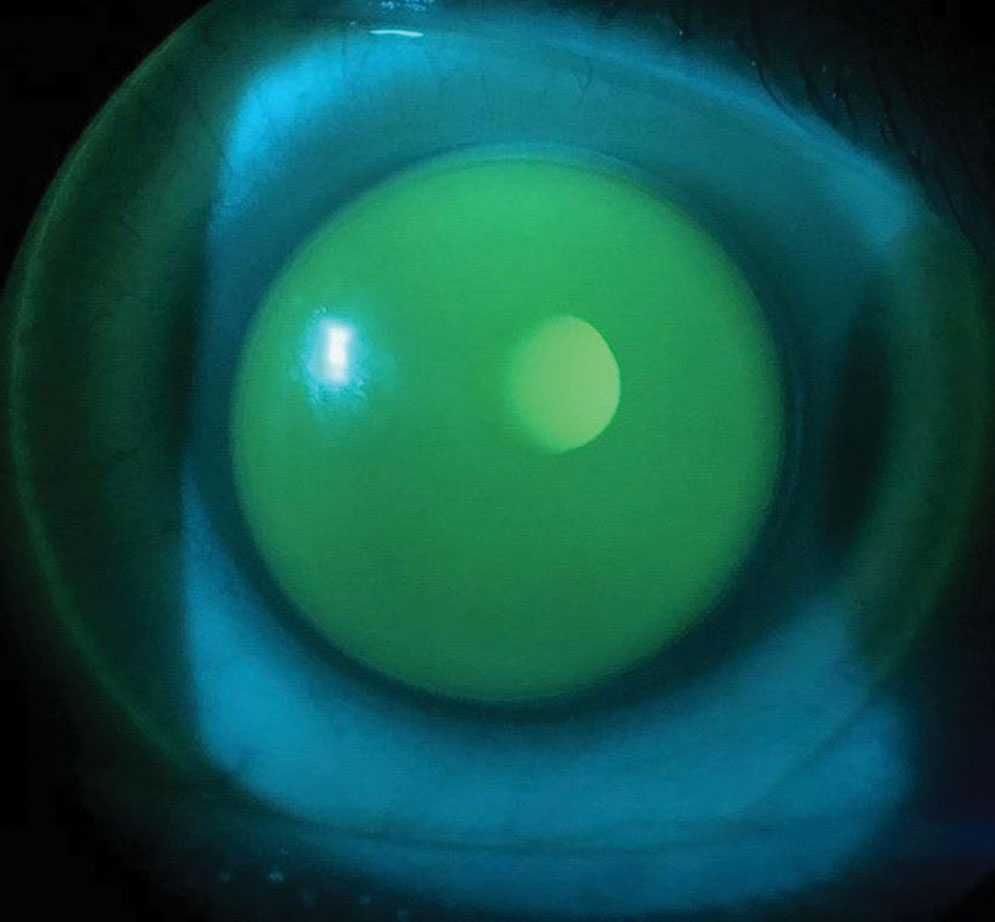 |
| Fig. 3. Sodium fluorescein demonstrates apical alignment, ILZ clearance and slight lens inferotemporal decentration. Click image to enlarge. |
Diagnostic Fitting
The initial UltraHealth FC diagnostic lens selected for the right eye was 155 vault/medium skirt/-1.50D. This lens showed significant fluorescein clearance at the central apex and a very loose fit overall. We immediately replaced the lens with the next lower vault design with parameters of 105 vault/medium skirt/-1.00D. This lens demonstrated fluorescein alignment, mild inner landing zone (ILZ) clearance and soft skirt alignment on the sclera (Figure 2a). Anterior segment OCT (AS-OCT) showed an apical tear film thickness of 150μm.
We believed we could achieve an even greater alignment fit with the lowest vault available in the diagnostic set, so we inserted a 55 vault/medium skirt/plano lens and determined this was the most appropriate vault for the patient’s right eye (Figure 2b). An overrefraction of -0.25D corrected his vision to 20/20. The finalized contact lens parameters were 55 vault/flat skirt/-0.25D.
During the diagnostic fitting of the patient’s left eye, the 105 and 155 vaults both showed “lens crash,” or heavy central bearing on slit lamp exam and AS-OCT. The 205 vault/medium skirt/-2.50D diagnostic lens demonstrated apical clearance over the cornea and limbus. There was good centration and tear film alignment in the ILZ (Figure 2c). However, after 30 minutes of lens settling, the patient noted ocular irritation and foreign body sensation. The nasal ILZ had “crashed” onto the peripheral cornea, and the temporal ILZ showed minimal tear layer vault (Figure 2d). With an overrefraction of -1.50D, the patient’s left eye corrected to 20/20. After adjusting for the vertex distance and lens crash on the ILZ, we ordered lens parameters of 205 vault/steep skirt/-3.75D.
-3.jpg) |
| Fig. 4. (A) Apical “lens crash,” or lens bearing, on AS-OCT after one hour of lens settling. (B) Peripheral ILZ bearing nasally (left) and temporally after one hour of lens settling. (C) The sodium fluorescein pattern shows faint central bearing that starkly contrasts with overt lens crash. Click image to enlarge. |
Dispensing
At the dispense visit, the patient had apical alignment, adequate lens centration and ILZ clearance OD (Figure 3). While the lens was decentered slightly inferotemporally, the patient stated he was satisfied with the visual acuity and comfort the lens offered, so further revision was not indicated. A case, however, could be made to flatten the skirt to lower the ILZ clearance, but that would increase the risk of even greater lens decentration and movement on blink.
Fluorescein showed apical clearance to pooling OS. The patient reported the lens was comfortable and read the 20/20 line without an overrefraction. However, after the lens settled for an hour, we discovered apical “lens crash” with ILZ bearing at both the nasal and temporal limbus (Figures 4a and 4b). Interestingly, the fluorescein pattern showed light apical thinning rather than frank or heavy bearing (Figure 4c). The proportion of the soft skirt and ILZ support was not ideal, so we revised to 255 vault/flat skirt/-4.75D.
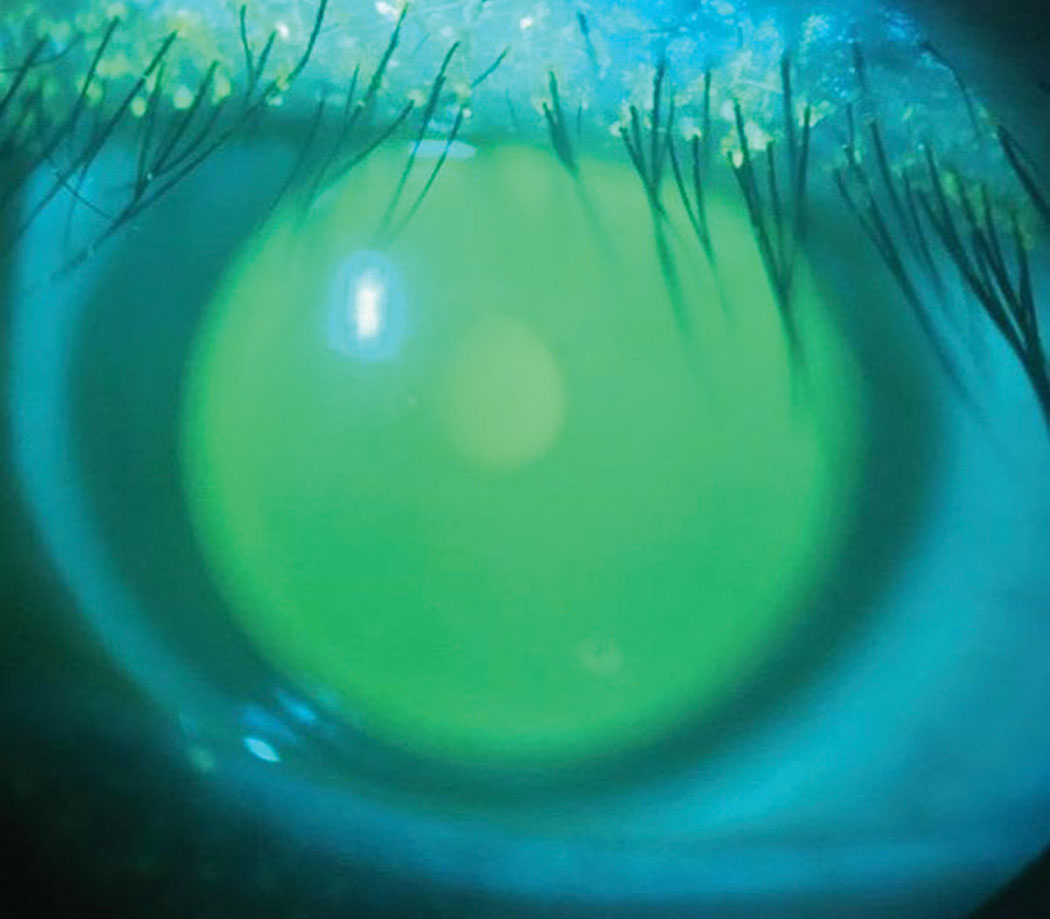 |
| Fig. 5. Fluorescein indicates central alignment without bearing or pooling of the ILZ. Click image to enlarge. |
This lens demonstrated apical alignment without any areas of touch or bearing, with the ILZ demonstrating ideal feather thinning and the soft skirt showing scleral alignment without edge fluting or tightness (Figure 5). AS-OCT demonstrated ideal tear film alignment of the lens and cornea after four to five hours of lens wear (Figure 6). The tear layer on the nasal ILZ showed satisfactory clearance with ideal soft lens cushion support. Temporally, the lens and soft skirt junction showed an optimal 80/20 relationship between the soft cushion of the skirt and the alignment of the ILZ tear layer (Figure 7).
The patient reported good comfort after 30 minutes of wear and read the 20/20 line without an overrefraction, so we were satisfied with the outcome, dispensed the lens to the patient and asked him return for a follow-up visit. We recommended preservative-free 0.9% saline for lens insertion with a DMV contact lens handler and BioTrue multipurpose solution for lens disinfection and storage.
Follow-up
The patient returned one week later after having worn his lenses at least six hours. He had been using preservative-free 0.9% saline for insertion and BioTrue (Bausch + Lomb) for disinfection and storage as instructed without any adverse symptoms.
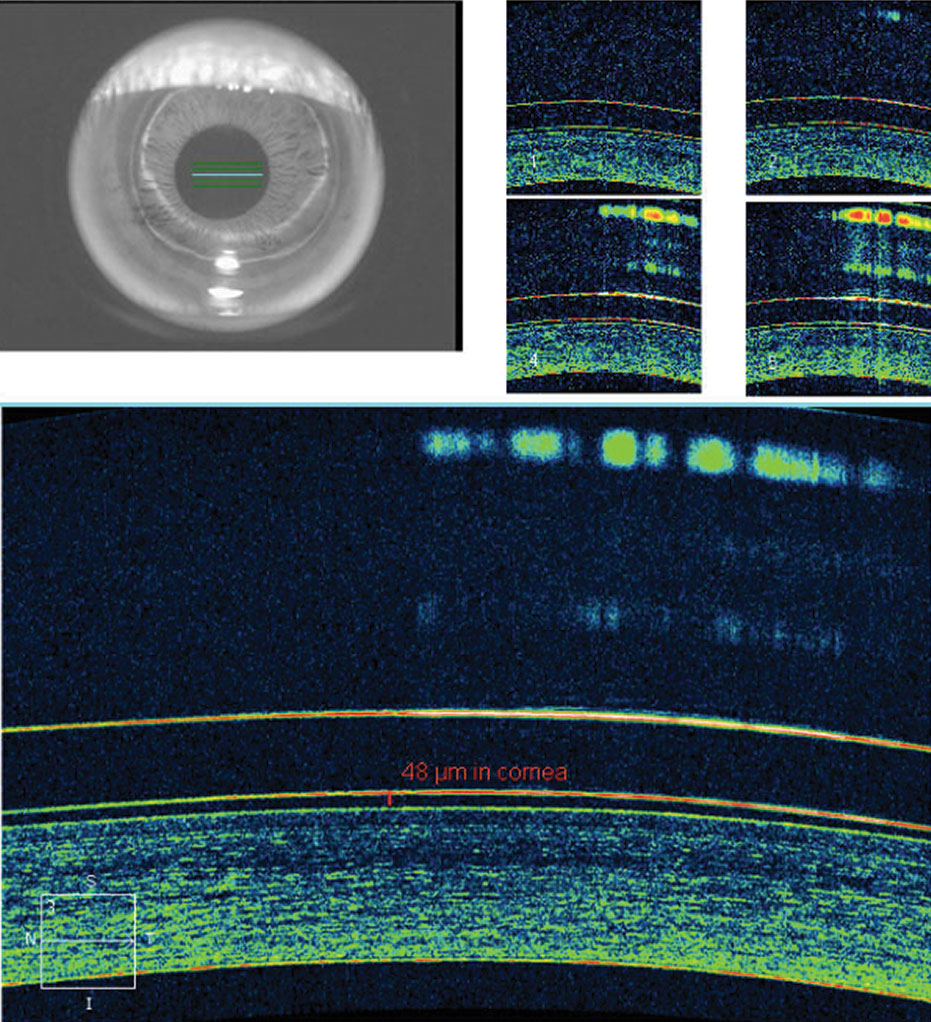 |
| Fig. 6. AS-OCT demonstrates ideal tear film alignment over the central cornea with good lens centration after a few hours of wear. Click image to enlarge. |
With the lenses, visual acuity was 20/20 with a plano overrefraction in each eye. Fluorescein showed ideal central alignment and light thinning in the ILZ on both eyes. though he still experienced minor lens awareness from time to time, he has been able to wear the lenses comfortably for at least eight hours each day.
We were happy with the fit, comfort and vision and asked him to return in a year, or sooner if he developed any ocular or visual issues. Note that despite the similar oblate Ks in both eyes, the final lens parameters were quite distinct from each other. The left eye required a much taller vault than the right, which highlights the importance of vaulting the central cornea and aligning to the peripheral cornea and sclera.
Discussion
A team of researchers found myopic regression can develop as early as three months in post-LASIK patients.5 Post-LASIK myopic regression is multifactorial in nature. Although its exact cause is unclear, a study determined that flap unevenness due to poor microkeratome placement may contribute.6 Similarly, a study investigating the prevalence of myopic regression in various flap-based surgeries reported more patients with mechanical microkeratome LASIK experienced myopic regression.7
Another study reviewed myopic regression following photorefractive keratectomy and determined that refractive treatments over -5.00D, optic zone treatment diameters under 6mm and unstable fixation during laser ablation all contributed to myopic progression.8 Other investigators confirmed that eyes treated within 6mm of the optic zone had a higher incidence of myopic regression.9
Several other factors have been documented to contribute to post-LASIK refractive regression. Corneal hysteresis (i.e., the cornea’s ability to absorb pressure) is another possible contributing factor. Lower corneal hysteresis positively correlates with increasing degrees of myopia.10 Other researchers observed a significantly higher frequency of myopic regression in cases with less than 350μm of residual stromal bed.9 Post-LASIK dry eye was also shown to increase the prevalence of myopic regression.4
-4.jpg) |
| Fig. 7. Ideal 80/20 support of the soft cushion of the contact lens with tear alignment posterior to the ILZ nasally (left) and temporally after settling. Click images to enlarge. |
The most common complication of LASIK is dry eye, with virtually all patients developing some degree of dryness during the post-op phase.11 The prevalence in the early post-op period has been reported as high as 95%.11 Not surprisingly, refractive surgeons have reported dry eye as the primary post-op complication.1
Multiple theories have been proposed about the pathophysiology of post-LASIK dry eye. The most common is iatrogenic corneal nerve damage during creation of the flap and ablation of the stromal tissue from the excimer laser.11 Loss of conjunctival goblet cells has also been documented after LASIK surgery due to direct damage from the high-pressure suction device used during creation of the corneal flap.11 Post-op inflammatory changes may also contribute.12 Elevated levels of matrix metalloproteinase-9, a pro-inflammatory proteolytic enzyme produced by stressed epithelial cells and neutrophils, have been measured in the tear film in post-op patients.13 This marker is responsible for prolonging healing, exacerbating dry eye symptoms and causing post-op haze.
The importance of providing specialty lens fittings for patients with post-op corneas cannot be overstated. As they are typically limited in their surgical enhancement options and generally have active lifestyles, it is crucial to offer specialty options if soft lenses provide a poor fit. It is equally important to develop a robust skillset in performing and troubleshooting these challenging fits, given the multitude of variables associated with post-LASIK regression.
Dr. Chung practices at the North Florida/South Georgia Veterans Health Administration, where he teaches externs and residents and serves as the lead contact lens fitting specialist.
Dr. Wong practices at the Malcom Randall VA Medical Center in Gainesville, FL, where he has worked in both the optometry and compensation/pension services.
Dr. Houser practices in Southwest Florida. She graduated from the NSU College of Optometry and completed a residency in ocular disease, low vision and specialty contact lenses at the Malcom Randall VA Medical Center.
1. Lim SA, Park Y, Cheong YJ, et al. Factors affecting long-term myopic regression after laser in situ keratomileusis and laser-assisted subepithelial keratectomy for moderate myopia. Korean J Ophthalmol. 2016;30(2):92-100. 2. Alió JL, Felipe S, Alessandro A, et al. Laser in situ keratomileusis for -6.00 to -18.00 diopters of myopia and up to -5.00 diopters of astigmatism: 15-year follow-up. J Cataract Refract Surg. 2015;41(1):33-40. 3. Albiertz JM, Lenton LM, McLennan SG. Chronic dry eye and regression after laser in situ keratomileusis for myopia. J Cataract Refract Surg. 2004;30(3):675-84. 4. Shtein RM. Post-LASIK dry eye. Expert Rev Ophthalmol. 2011;6(5):575-82. 5. Ikeda T, Shimizu K, Igarashi A, et al. Twelve-year follow-up of laser in situ keratomileusis for moderate to high myopia. Hindawi: Biomed Resear Int. May 17, 2017. [Epub ahead of print]. 6. Lin MY, Chang DCK, Hsu WM, et al. Cox proportional hazards model of myopic regression for laser in situkeratomileusis flap creation with a femtosecond laser and with a mechanical microkeratome. J Cataract Refract Surg. 2012;38(6):992-9. 7. Zhou J, Gu W, Li S, et al. Predictors affecting myopic regression in -6.0D to -10.0D myopia after laser-assisted subepithelial keratomileusis and laser in situ keratomileusis flap creation with femtosecond laser-assisted or mechanical microkeratome-assisted. Int Ophthalmol. 2020;40:213-25. 8. Mohammadi SF, Nabovati P, Mirzajani A, et al. Risk factors of regression and undercorrection in photorefractive keratectomy: a case-control study. Int J Ophthalmol. 2015;8(5):933-7. 9. Chen YI, Chien KL, Wang IJ, et al. An interval-censored model for predicting myopic regression after laser in situ keratomileusis. Invest Ophthalmol Vis Sci. 2007;48:3516-23. 10. Qiu K, Lu X, Zhang R, et al. Corneal biomechanics determination in healthy myopic subjects. Hindawi: J Ophthalmol. July 21, 2016. [Epub ahead of print]. 11. Hovanesian JA, Shah SS, Maloney RK. Symptoms of dry eye and recurrent erosion syndrome after refractive surgery. J Cataract Refract Surg. 2001;27(4):577-84. 12. Solomon KD, Holzer MP, Sandoval HP, et al. Refractive surgery survey 2001. J Cataract Refract Surg. 2002;28(2):346-55. 13. Chan TCY, Ye C, Chan KP, et al. Evaluation of point-of-care test for elevated tear matrix metalloproteinase 9 in post-LASIK dry eyes. Br J Ophthalmol. 2016;100(9):1188-91. |


