 |
 |
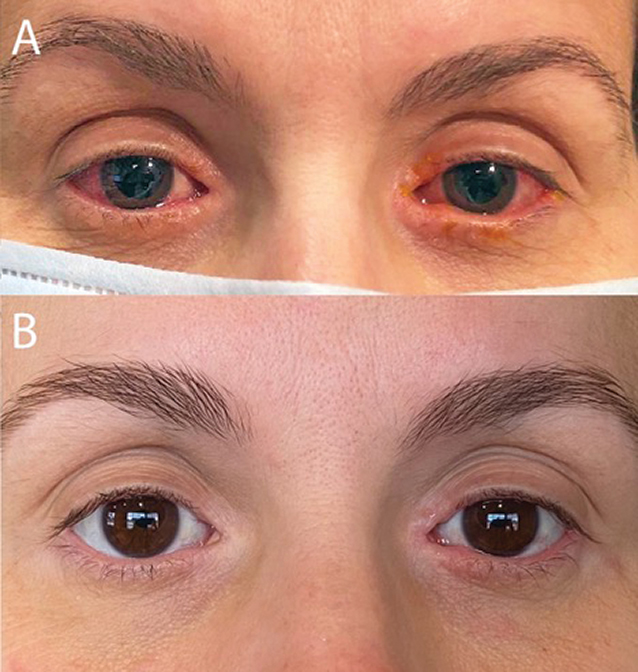
A 42-year-old woman requested a virtual eye care visit for symptoms of chronic redness, dryness, tearing and itching of both eyes (Figure 1A). She had been treated in the past with OTC topical antihistamine and mast cell stabilizer eye drops without resolution of symptoms. She was using topical triamcinolone 0.1% cream nightly for periocular erythema and topical neomycin-polymixin B-dexamethasone eye drops once per day, both of which improved but did not resolve symptoms with the recommended dosage. She was also using artificial tears daily and Lumify (brimonidine tartrate 0.025%, Bausch + Lomb) as needed. Her medical history was significant for atopic dermatitis, for which she was prescribed Dupixent (dupilumab, Sanofi and Regeneron Pharmaceuticals) two years prior to presentation.
A tentative diagnosis of dupilumab-associated ocular surface disease was made, and the patient was asked to visit the clinic for a thorough eye exam. Use of topical steroids and Lumify was stopped until evaluation. On examination, her best-corrected visual acuity was 20/20 in each eye with no afferent pupillary defect. Her extraocular motilities and confrontation fields were full, and intraocular pressures (IOP) were 17mm Hg OD and 18mm Hg OS. Her slit lamp exam revealed bilateral periocular erythema and meibomian gland dysfunction with thickened and mildly keratinized lid margins. There was 2-3+ diffuse bulbar hyperemia with 3+ papillae in both eyes. There were multiple white focal eosinophilic aggregates at the limbus and a slightly reduced tear breakup time of seven to eight seconds bilaterally. Her dilated fundus exam was unremarkable.
 |
|
Fig. 1. (A) Initial clinic visit reveals bilateral conjunctival injection, epiphora and blepharitis. (B) Patient-provided photograph with significant improvement three months after initiation of dedicated ophthalmic therapy. Click image to enlarge. |
The patient was started on prednisolone acetate 1% eye drops four times daily, Restasis (cyclosporine 0.05%, Allergan) twice daily, hypochlorous acid eyelid cleanser twice daily and warm compresses once per day. Upon return, her clinical findings and symptoms had improved significantly, but IOPs measured 26mm Hg OD and 29mm Hg OS. Due to steroid-induced ocular hypertension, prednisolone 1% was discontinued in favor of fluorometholone 0.1%. Dorzolamide 2% twice daily was added temporarily.
At her next follow-up, symptomatic improvement had been maintained and her IOP had improved to the mid-teens. The fluorometholone eye drops were tapered to once daily and the patient continues to use cyclosporine twice daily, eyelid scrubs and warm compresses once daily, and artificial tears as needed. With this dedicated ophthalmic management, she has been able to continue Dupixent (Figure 1B).
What Is Dupixent?
A first-in-class medication for difficult-to-manage allergic or inflammatory conditions, Dupixent was FDA-approved for moderate-to-severe atopic dermatitis in 2017. Since then, the drug’s indications have been extended to include moderate-to-severe asthma and inadequately controlled chronic rhinosinusitis with nasal polyposis.1 It is currently being evaluated for use in eosinophilic esophagitis. Dupilumab is a monoclonal antibody that serves as a dual inhibitor of interleukin-4 (IL-4) and interleukin-13 (IL-13) signaling pathways. This mediation is administered by subcutaneous injection and is typically dosed every two weeks. It has been approved for use in individuals as young as six years of age.
Side Effects
So why, as optometrists, do we need to know about this drug? Potential adverse effects include ocular side effects such as conjunctivitis and keratitis. According to a literature review, conjunctivitis was seen in anywhere from 9% to 38% of patients.2-4 The higher incidence in more recent studies may be due to a selection bias, smaller study sizes, increased recognition of disease or exacerbations of previously undiagnosed ocular surface disease.
The pathophysiology behind the development of ocular surface side effects related to dupilumab is still a point of ongoing research. Multiple studies have shown a link between the IL-13 signaling pathway and the proper maintenance of goblet cells. With IL-13 blockage, conjunctival goblet cell density is drastically decreased, which can lead to increased ocular surface inflammation, decreased tear film stability and epithelial barrier dysfunction.5,6 Literature has also demonstrated an increase in OX40 ligand activity, eosinophilia and Demodex infestations, which can all negatively impact the ocular surface.2
 |
|
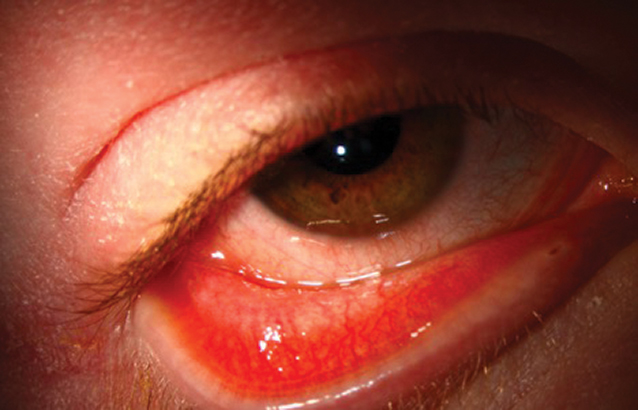
Fig. 2. This 17-year-old male with severe atopic dermatitis was started on dupilumab. There was a marked skin improvement which he called “life-changing,” but shortly thereafter he developed itching, redness, epiphora, photophobia and ocular discomfort. The clinical exam revealed bulbar and palpebral conjunctival injection with a mixed papillary and follicular response, punctate erosions and filamentary keratitis. Click image to enlarge. |
A history of ocular surface disease—such as atopic keratoconjunctivitis (AKC)—and more severe atopic dermatitis prior to treatment with dupilumab are considered to be the most common risk factors in the development of dupilumab-associated ocular surface disease.2 Though there is often phenotypic overlap in the clinical diagnoses of this disease and AKC, the histopathologic findings are quite different. AKC and allergic conjunctivitis typically manifest increased density of goblet cells and mucus production (i.e., ropy discharge), whereas dupilumab reveals the opposite.
It’s worth mentioning that dupilumab therapy was not associated with an increased incidence of conjunctivitis compared with the placebo when used for other conditions, such as asthma, rhinosinusitis and eosinophilic esophagitis.7 Because these last three conditions are not directly associated with ocular surface disorders themselves, there may be an avenue for further research here.
Treatment
Since its debut, use of this drug has continued to climb due to its clinical success and increasing list of indications. Many severe cases of atopic dermatitis otherwise recalcitrant to treatment have shown significant skin improvement with dupilumab. Therefore, these patients are often reluctant to discontinue this life-changing therapy. In most cases, management of the ocular surface complications can allow a patient to continue their systemic treatment.
What to Look ForThe presentation of ocular surface and periocular effects varies drastically between patients, so it is important to be aware of all possible findings. Clinical ocular findings include conjunctival hyperemia, follicular conjunctivitis, limbal nodules, filamentary keratitis, dry eye, blepharitis and Demodex infestation. More severe complications include conjunctival cicatricial changes, madarosis, punctal stenosis, cicatricial ectropion and limbal stem cell deficiency.8,9 |
After diagnosing dupilumab-associated ocular surface disease, initiate treatment. One study suggested a treatment algorithm in which mild cases may respond well to topical ocular lubricants and antihistamine-mast cell stabilizers alone.8 Moderate-to-severe cases will likely require topical steroid drops as a first-line treatment option. The researchers also recommended starting a steroid four times daily until symptoms resolve, then gradually tapering by one drop every two weeks to the lowest dose tolerated. Patients need to be advised of the ocular risks of steroids, which include steroid-induced ocular hypertension or glaucoma and cataract formation. Therefore, long-term steroids should be avoided if possible, especially for children or those who have these adverse effects. As seen in our patient, other steriod-sparing agents may also be used successfully in this population of patients. Studies have shown improvement in with topical cyclosporine, lifitegrast and tacrolimus.10
Moving Forward
It’s important to recognize that this class of medications is not going away. In fact, ongoing research has delivered promising results so far for at least two other monoclonal antibodies that also block IL-13 signaling pathways. Both lebrikizumab and tralokinumab are currently in clinical trials, indicated for atopic dermatitis.11,12
As primary eye care providers, we should be on the front lines of managing the ocular complications of these medications. Optometrists should consider reaching out to their local dermatology practices and offering their clinical expertise to help manage these patients. This new drug class has been life-altering for so many individuals—we can’t lose sight of the fact that our profession can help them in more ways than one.
1. Sanofi Genzyme, R. Dupilumab (Dupixent) Injection. 2020; Available at www.dupixenthcp.com. 2. Popiela MZ, Ramez B, Turnbull AMJ, et al. Dupilumab-associated ocular surface disease: presentation, management and long-term sequelae. Eye. January 28, 2021. 3. Barbe J, Poreaux C, Remen T, et al. Prevalence of ocular disease during dupilumab treatment for atopic dermatitis: a bicentric retrospective comparative cohort study. Int J Dermatol. May 26 2021. 4. Faiz S, Giovannelli J, Podevin C, et al. Effectiveness and safety of dupilumab for the treatment of atopic dermatitis in a real-life French multicenter adult cohort. J Am Acad Dermatol. 2019; 81(1):143-51. 5. Tukler Henriksson, J., et al., IL-13 stimulates proliferation and expression of mucin and immunomodulatory genes in cultured conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2015; 56(8):4186-97. 6. Bakker DS, Ariens LFM, Lujik CV, et al, Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br J Dermatol, 2019; 180(5):1248-9. 7. Agnihotri G, Shi K, Lio PA. A clinician’s guide to the recognition and management of dupilumab-associated conjunctivitis. Drugs R D. 2019; 19(4):311-8. 8. Levine RM, Tattersall IW, Gaudio PA, King BA. Cicatrizing blepharoconjunctivitis occurring during dupilumab treatment and a proposed algorithm for its management. JAMA Dermatol. 2018; 154(12):1485-6. 9. Mehta, U. and M. Farid, Dupilumab induced limbal stem cell deficiency. Int Med Case Rep J. 2021; 14:275-8. 10. Nahum Y, Mimouni M, Livny E, et al. Dupilumab-induced ocular surface disease (DIOSD) in patients with atopic dermatitis: clinical presentation, risk factors for development and outcomes of treatment with tacrolimus ointment. Br J Ophthalmol. 2020; 104(6):776-9. 11. Simpson EL, Flohr C, Eichenfield LF, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: A randomized, placebo-controlled phase II trial (TREBLE). J Am Acad Dermatol. 2018; 78(5):863-71 e11. 12. Wollenberg A,Howell MD, Guttman-Yassky E, et al. Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J Allergy Clin Immunol. 2019; 143(1):135-41. |


