Fuchs’ dystrophy, a progressive corneal ailment, is characterized by the gradual deterioration and eventual demise of endothelial cells, leading to a significant reduction in their number and functionality. This decline in corneal endothelial cell density disrupts the pump mechanism responsible for maintaining corneal hydration. As a result, excess fluid accumulates in the cornea, leading to corneal edema. The buildup of fluid causes the cornea to swell, resulting in blurred and distorted vision.1,2
The underlying cause is not fully understood; however, it is believed to involve a complex interplay of both genetic and environmental factors. Several genes have been identified as potential contributors to Fuchs’ dystrophy. Notably, there seems to be a higher risk of the disease among individuals with a family history of the condition. This suggests that genetic predisposition plays a role in its development.1,2
Environmental factors are also believed to contribute to the onset and progression. Oxidative stress, which occurs when there is an imbalance between the production of free radicals and the body’s ability to neutralize them, is thought to play a role in the deterioration of endothelial cells. Additionally, excessive exposure to ultraviolet (UV) radiation, particularly from the sun, may also damage the corneal endothelium, further contributing to the disease process.1,2
It is important to note that Fuchs’ progresses slowly and typically affects individuals over the age of 50. The rate of progression and severity of symptoms can vary greatly between individuals. While there is no cure, there are treatment options available to alleviate symptoms and manage the condition. First-line treatments include topical distillation of hypertonic saline, topical steroids or artificial tears. This helps to reduce corneal swelling and improve vision. Moderate-to-severe Fuchs’ may require corneal transplantation, which involves replacing the affected cornea with a healthy donor cornea. There are many different methods, including Descemet’s stripping automated endothelial keratoplasty (DSAEK), Descemet’s membrane endothelial keratoplasty (DMEK) and others.1-3
Regular monitoring is crucial to assess the condition’s progression and determine the appropriate treatment approach. By closely managing the condition, it is possible to preserve vision and maintain a good quality of life for those affected by Fuchs’ dystrophy.
 |
|
A patient with Fuch’s dystrophy showing focal areas of swelling superotemporal and inferotemporal. Click image to enlarge. |
Symptoms and Progression
The endothelium plays a crucial role in maintaining corneal clarity and hydration. In the early stages of Fuchs’ dystrophy, the endothelial cells gradually decrease in number and function; this can lead to corneal swelling and various symptoms.1,2,4 As the disease progresses, the following symptoms may appear:
Blurred vision. This is the most common symptom. Because the endothelial cells are unable to pump excess fluid out of the cornea efficiently, blurred vision is often worse in the morning due to overnight fluid accumulation in the cornea.
Glare and halos. Increased sensitivity to light and the appearance of halos around lights are also common symptoms. This occurs because the irregular shape of the swollen cornea scatters light, causing visual disturbances.
Pain or discomfort. In advanced stages, corneal erosions or bullae (fluid-filled blisters) can develop on the corneal surface, causing significant pain, discomfort and vision problems.
Decreased visual acuity. Reduced sharpness and clarity of vision are more common symptoms, especially in low-light conditions. This is because the swollen cornea affects the way light enters and focuses on the retina, resulting in decreased visual acuity.
The progression of Fuchs’ dystrophy varies from person-to-person and is influenced by several factors, including age, genetics and environmental factors such as exposure to ultraviolet radiation and smoking.
Impact on Higher-Order Aberrations (HOAs)
These are optical imperfections in the eye that can significantly impact the quality of vision, even in individuals with normal visual acuity. HOAs cause distortions, such as glare, halos and decreased contrast sensitivity, particularly in low-light conditions.4
 |
|
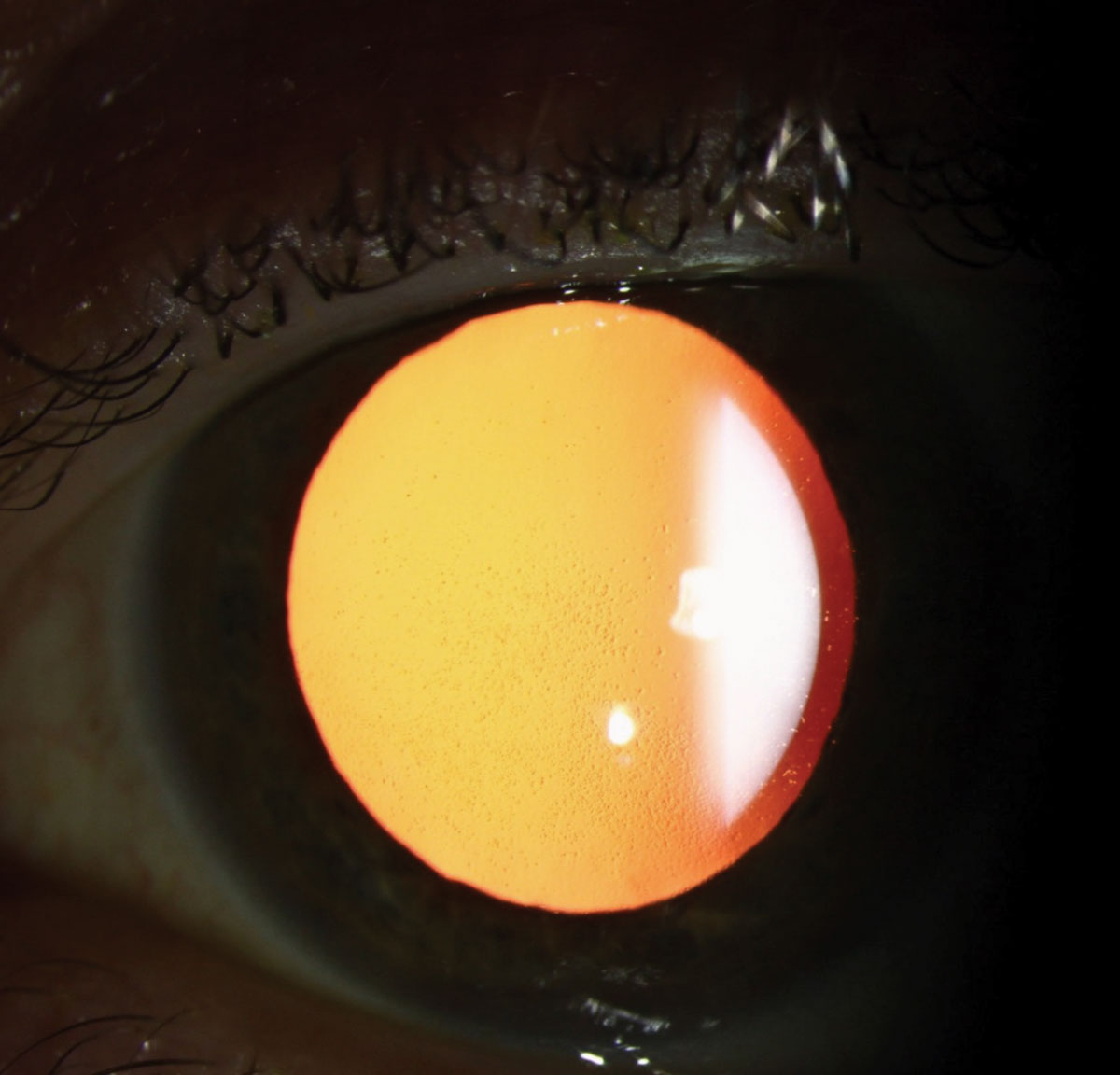
Retroillumination slit lamp photo showing corneal guttae. Click image to enlarge. |
The fluid accumulation in the cornea associated with Fuchs’ dystrophy alters its shape and refractive properties, leading to an increase in HOAs. This can further exacerbate the visual symptoms, making it challenging for patients to see clearly, especially in low-light conditions.4
Recent studies have investigated the relationship between Fuchs’ dystrophy and HOAs. These studies have consistently demonstrated a significant increase in HOAs in individuals with the condition compared to healthy individuals. The increase in HOAs was particularly pronounced in the early morning when corneal edema is typically most severe. Furthermore, the studies showed that the increase in HOAs correlated with the severity of the disease, suggesting that HOAs may serve as a potential biomarker for monitoring disease progression.4
Theses findings highlight the importance of considering HOAs in the management of Fuchs’ dystrophy. By understanding the impact of HOAs on vision, clinicians can better tailor treatment strategies to address the specific visual needs of individuals with Fuchs’ dystrophy. Additionally, monitoring HOAs over time is now possible with aberrometry and can provide valuable insights into disease progression and help guide treatment decisions.4
Further research is needed to explore the potential of HOAs as a diagnostic tool for Fuchs’ dystrophy and to investigate the impact of various treatment modalities on HOAs. This research may lead to the development of more effective treatments, ultimately improving the quality of life for individuals affected by this condition.
Diagnosis
A comprehensive eye examination is needed to make a diagnosis, which typically includes the following:
Slit-lamp examination may reveal corneal guttae on the endothelium, which can appear white or pigmented.
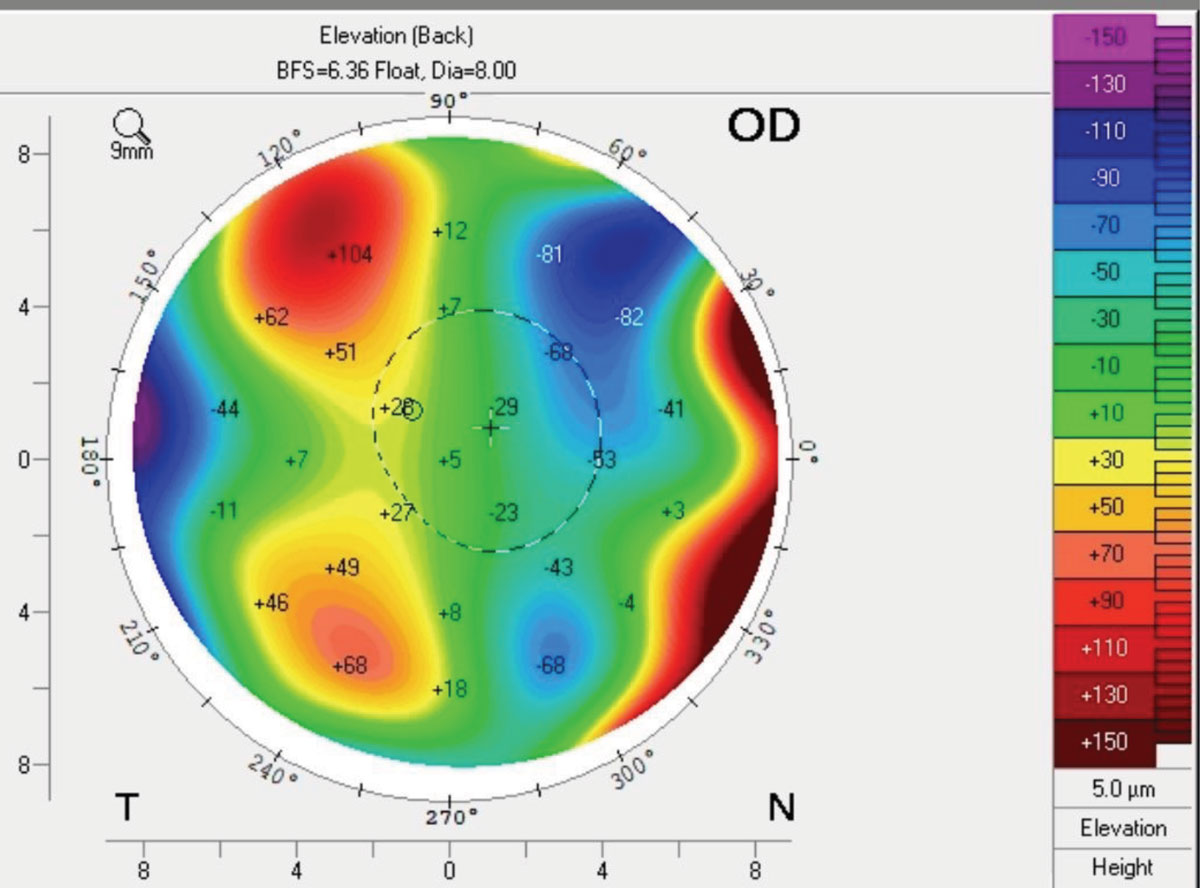
Corneal tomography may show irregular astigmatism and is also sensitive to intracorneal swelling by measuring pachymetry or back surface elevation (as the cornea will thicken with corneal swelling).
Endothelial cell count imaging measures the density of endothelial cells in the cornea. It can also provide qualitative analysis on the size and shape of the endothelial cells. Patients with Fuchs’ dystrophy will often show decreased endothelial cell count, along with pleomorphism and polymegethism with this type of imaging.1
Genetics
While the exact genetic mechanisms are not completely understood, research has identified several key genes and chromosomal regions implicated in its development:1-2
TCF4: This gene is the most frequently associated with Fuchs’ dystrophy, particularly in individuals of Caucasian descent. A specific mutation within this gene accounts for a significant proportion of cases.
COL8A2: Mutations in this gene are linked to an early-onset variant of Fuchs’ dystrophy, characterized by more rapid progression and the formation of corneal bullae. This gene is responsible for the production of type VIII collagen, crucial for supporting the corneal endothelium.
SLC4A11: This gene codes for a sodium borate cotransporter, and its mutations have been identified in individuals with late-onset Fuchs’ dystrophy. The exact role of this gene in corneal health is still under investigation.
ZEB1: This gene is involved in cell differentiation and has been associated with Fuchs’ dystrophy in some studies.
Inheritance patterns. Fuchs’ dystrophy can be inherited in an autosomal dominant pattern, meaning that only one copy of the mutated gene is sufficient to cause the condition. This is particularly evident in cases linked to COL8A2 mutations.
In some families, the inheritance pattern remains unclear, suggesting that other genetic or environmental factors may be involved. There are also documented cases that arise from spontaneous mutations, occurring in individuals with no family history of the disease.1-2
 |
|
Retroillumination slit lamp photo showing corneal guttae. Click image to enlarge. |
Management
Antioxidant therapy is a promising approach to counteract oxidative stress in Fuchs’ dystrophy. These compounds neutralize reactive oxygen species, reducing oxidative damage and preserving cellular function. Topical application of antioxidant agents to the cornea may help protect endothelial cells from oxidative stress. For example, N-acetylcysteine is a precursor to glutathione and has been explored for its ability to enhance the antioxidant capacity of corneal endothelial cells. Systemic administration of antioxidants such as vitamins C and E, alpha-lipoic acid and coenzyme Q10 may provide broader protection against oxidative damage.
Mitochondrial dysfunction plays a central role in Fuchs’ dystrophy-related oxidative stress. Compounds such as mitoquinone (MitoQ) specifically target the mitochondria to reduce reactive oxygen species (ROS) production and improve mitochondrial function. These targeted therapies may offer more precise protection for endothelial cells.1,2
Advances in the genetic understanding of Fuchs’ dystrophy offer potential for gene therapy approaches aimed at reducing oxidative stress in endothelial cells. Therapies targeting the TCF4 gene or other genetic contributors may help modulate the stress response pathways and enhance cellular resilience to oxidative damage. Gene editing (such as CRISPR/Cas9) allows for precise correction of genetic mutations, such as those in the TCF4 gene. Correcting these mutations could improve the oxidative stress response in endothelial cells, potentially halting or slowing disease progression.1,2
Several pharmacological agents targeting oxidative stress and cellular protection are being explored. Rho-kinase inhibitors have shown promise in promoting endothelial cell survival and reducing oxidative damage, which may enhance endothelial cell resilience and improve corneal clarity in patients with early-stage disease.1,2
One recent advancement is the development of a novel ubiquinol/γ-cyclodextrin (γ-CD) complex to improve the solubility, stability and delivery of ubiquinol for ophthalmic applications. Ubiquinol, the reduced form of coenzyme Q10, is a potent antioxidant known to suppress ferroptosis, a form of cell death driven by iron-dependent lipid oxidation. However, ubiquinol’s poor water solubility and instability have hindered its therapeutic application.5
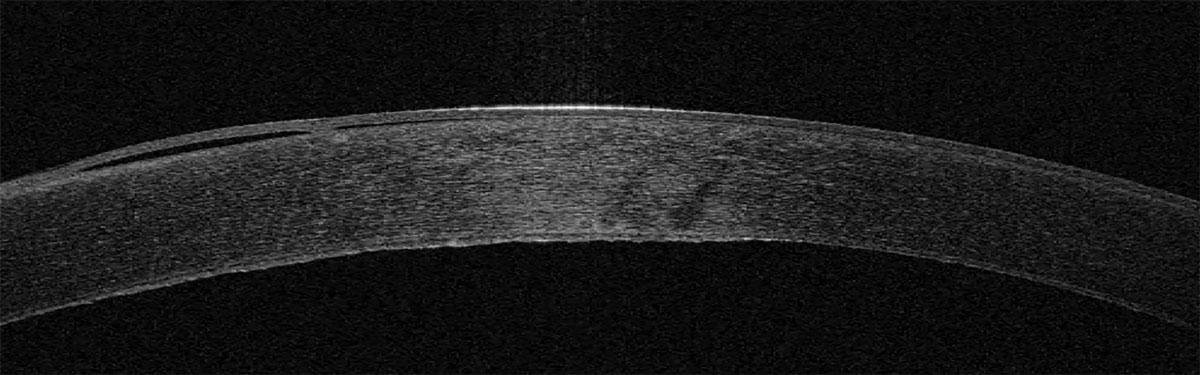 |
Anterior segment OCT shows the irregular corneal endothelium in a patient with Fuchs’ dystrophy. Click image to enlarge. |
The authors conducted molecular docking studies, enabling a significant portion of ubiquinol’s hydrophobic tail and head group characterized by both hydrogen bonding and hydrophobic forces, contributing to the complex’s stability. They found the following5:
Enhanced stability. Complexed ubiquinol demonstrated significantly higher stability in various aqueous media relevant to ophthalmic use, including: corneal transplant storage mediums, intraocular irrigation solution and artificial tears—compared to free ubiquinol.
Improved ROS scavenging. The complex effectively lowered ROS levels in both lung epithelial cells, a model with high basal ROS production and human corneal endothelial cells. This effect was more pronounced with the complex, rather than with free ubiquinol.
Inhibition of lipid peroxidation and ferroptosis. The complex effectively inhibited ferroptosis in cells. Importantly, the complex completely protected these cells from erastin-induced ferroptosis, while free ubiquinol did not. Similar results were observed with RSL3, another ferroptosis inducer.
Enhanced corneal penetration. The authors demonstrated that the complex facilitated transcorneal penetration, enabling delivery to the corneal endothelium. Similarly, the ubiquinol complex showed superior penetration and higher retention in the corneal endothelium compared to free ubiquinol in both topical application and storage media models.
It was concluded that the ubiquinol complex holds great promise for various ophthalmic applications, including:
Preservation of donor corneal tissue. Supplementing corneal storage media with the complex could improve the viability and quality of donor corneas, potentially increasing tissue availability and transplant success rates (oxidative stress is considered a major factor contributing to cell death and decreased graft survival).
Treatment of Fuchs’ dystrophy. As a topical therapy, the complex could help protect corneal endothelial cells from oxidative damage, potentially slowing disease progression and reducing the need for corneal transplantation.
This research highlights the potential of using cyclodextrin complexes to improve the delivery and therapeutic efficacy of ubiquinol. Further research is needed to determine the optimal dose and long-term effects of this novel formulation in clinical settings.5
Lifestyle Modifications
Reducing exposure to environmental factors that exacerbate oxidative stress may also be beneficial in managing Fuchs’ dystrophy. UV radiation is a known source of oxidative stress in the eye. Wearing UV-blocking sunglasses and avoiding excessive sun exposure may help reduce the oxidative burden on corneal endothelial cells. A diet rich in antioxidants including leafy greens, fruits and nuts, may help support the body’s natural defenses against oxidative stress. Systemic administration of antioxidants such as vitamins C and E, alpha-lipoic acid and coenzyme Q10 may provide broader protection against oxidative damage by improving the overall redox state of the body.2
Surgical Options
These treatments aim to replace the damaged endothelial cells that maintain corneal clarity. DMEK is the preferred technique, replacing the diseased Descemet’s membrane and endothelium with a healthy donor graft. This method offers excellent visual outcomes and faster recovery with low rejection rates. DSAEK replaces a slightly thicker layer of the cornea and may be suitable for patients who aren’t ideal candidates for DMEK. Penetrating keratoplasty, a full-thickness corneal transplant, is rarely used for Fuchs’ dystrophy now, and comes with the longest recovery time.
There is also a newer, less invasive option being studied called Descemetorhexis without Endothelial Keratoplasty, where the diseased Descemet’s is removed to allow healthy cells to regenerate, but long-term outcomes are still being evaluated.1,3
Takeaways
While the exact cause remains elusive, a complex interplay of genetic and environmental factors is thought to contribute to Fuchs’ onset and progression. The disease’s hallmark is the gradual loss of corneal endothelial cells, leading to corneal edema and visual impairment. Fortunately, recent advancements in research offer a glimmer of hope. The development of a novel ubiquinol formulation shows promise in protecting corneal endothelial cells and potentially slowing disease progression. This, coupled with ongoing genetic research and the exploration of new drug therapies, signifies a turning point in the fight against Fuchs’ dystrophy. As these promising avenues continue to be explored, the future holds the potential for improved treatment options and enhanced outcomes for individuals affected by this challenging condition
Dr. Noyes completed his Doctor of Optometry degree at the University of Houston College of Optometry, followed by a residency in Cornea & Contact Lens/Ocular Disease at The Ohio State University Havener Eye Institute. He is involved in the Fellowship of the American Academy of Optometry and Fellowship of the Scleral Lens Society. He currently works as a clinical assistant professor at the University of Iowa department of ophthalmology in the specialty contact lens department and is a member of the Scleral Lens Society fellowship committee.
1. Altamirano F, Ortiz-Morales G, O’Connor-Cordova MA, et al. Fuchs’ endothelial corneal dystrophy: an updated review. Int Ophthalmol. 2024;44(1):61. 2. Ong Tone S, Kocaba V, Böhm M, et al. Fuchs’ endothelial corneal dystrophy: the vicious cycle of Fuchs’ pathogenesis. Prog Retin Eye Res. 2021;80:100863. 3. Garcerant D, Hirnschall N, Toalster N, et al. Descemet’s stripping without endothelial keratoplasty. Curr Opin Ophthalmol. 2019;30(4):275-85. 4. Kobashi H, Kamiya K, Shimizu K. Factors influencing visual acuity in Fuchs’ endothelial corneal dystrophy. Optom Vis Sci. 2018;95(1):21-6. 5. Naguib YW, Saha S, Skeie JM, et al. Solubilized ubiquinol for preserving corneal function. Biomaterials. 2021;275:120842. |


