 |
Our 63-year-old male patient was dealing with resolving pseudodendrites. One month later, the same patient called in for an emergency visit. He reported that his left eye had worsened over the weekend and he was experiencing more pain and redness. He was still taking valacyclovir 1g PO daily and had slowly tapered off the steroid eye drops. His uncorrected visual acuity OS was 20/60-; intraocular pressure (IOP) was 10mm Hg. A corneal slit lamp exam revealed a LASIK flap, guttata, no keratic precipitates, central and peripheral nummular stromal infiltrates and early scarring. There were no signs of cells in the anterior chamber, and the posterior views were unremarkable. He was diagnosed with zoster stromal keratitis, also known as nummular keratitis. The patient was told to increase his Valtrex (valacyclovir, GlaxoSmithKline) use to three times a day as well as to restart his prednisolone drops twice a day OS.
Back in the Chair
| Read part one of this case from the March/April RCCL issue here. |
One week later, the patient was back in my chair and his nummular presentation was fully resolved. He struggled with side effects of fatigue from the high amount of valacyclovir but felt he did not want to reduce the dosage. It was decided to do an exceptionally long taper of the prednisolone that would span over one month. Within that month, the patient was seen again and the Valtrex was reduced to BID, while prednisolone was kept at QD. Another week later, the patient called in again with an urgent request to be seen due to blurred vision OS. He was seen in the office and it was confirmed this was his third recurrence with nummular keratitis present.
To summarize, this patient had a third instance of worsening vision, symptoms and keratitis while still on Valtrex PO BID and prednisolone QD within a span of four months. At this point, we increased his prednisolone back to QID and reached out to his primary care provider about long-term use of high dosages of Valtrex.
Since the last attack, the patient had been able to reduce Valtrex PO to QD and tapered to prednisolone TID; BID is where we decided to keep him. His keratitis resolved and his corneal scar had reduced dramatically. This leaves the question of how to manage him long-term.
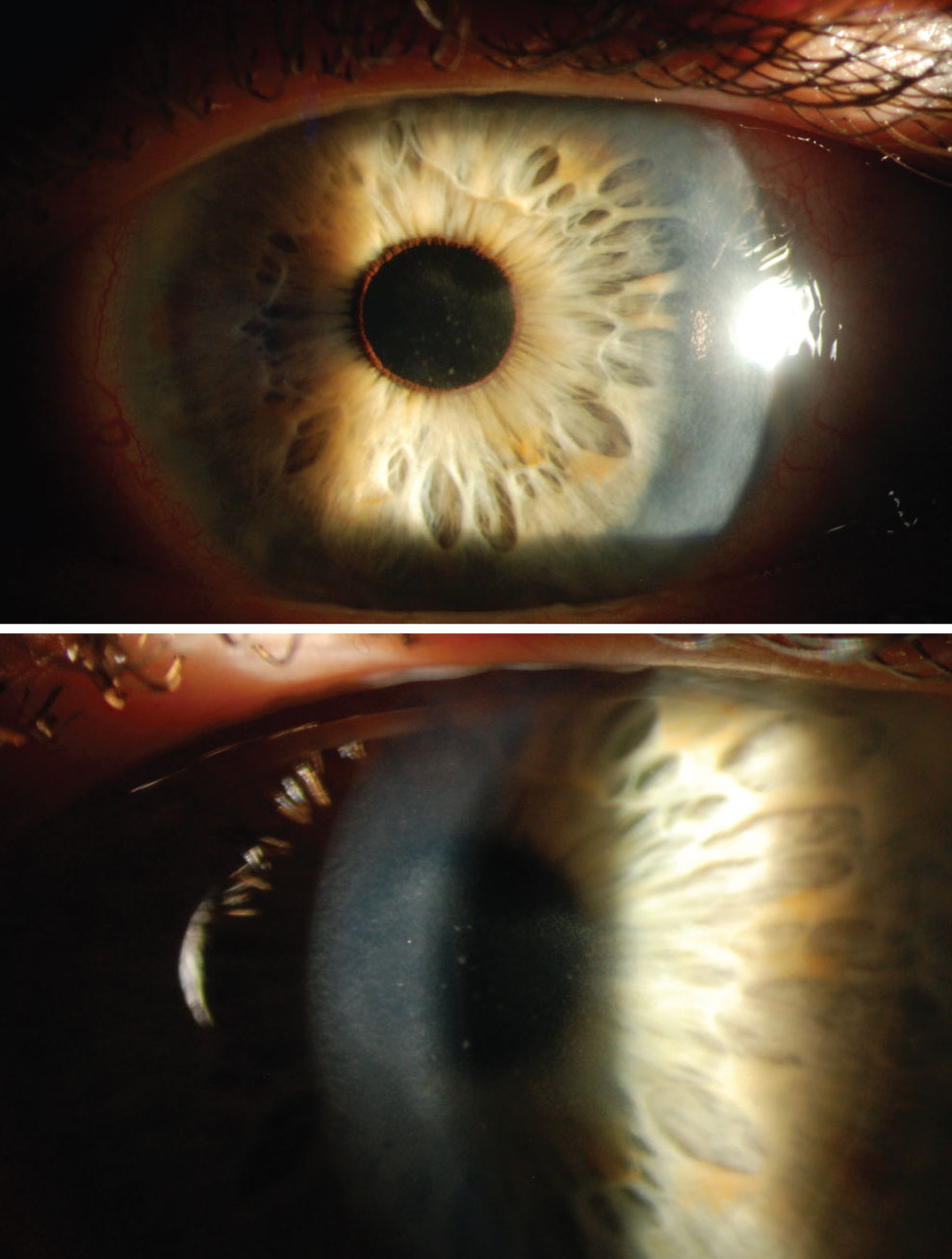 |
|
The patient presenting with nummular stromal keratitis caused by HZO. Click image to enlarge. |
HZO
Herpes zoster ophthalmicus (HZO) is a manifestation of the herpes zoster virus and is present in 20% of all herpes zoster cases. It is a severe variant of shingles and occurs when the immune system is weakened, causing the virus responsible for chicken pox to reactivate. HZO can affect all parts of the eye, and ocular onset usually occurs two to four weeks after the first appearance of a rash.
Initial treatment with antiviral therapy reduces the risk of chronic ocular complications by 20% to 30%. Initial treatment for HZO ideally begins within the first 72 hours of symptom onset. Antiviral options include acyclovir 800mg orally five times a day for at least seven days, valacyclovir 1000mg orally every eight hours for at least seven days or famciclovir 500mg orally three times per day for at least seven days. If the patient is immunocompromised, intravenous acyclovir or foscarnet may be necessary. When considering IV antivirals, keep in mind that there is a higher association with acute kidney injury and renal failure due to intratubular crystal precipitation and rapid excretion in the urine.1 Preventative treatment includes the vaccine, which can reduce the risk of viral reactivation; however, this only lasts for about eight ears.
The treatment and management of recurrent disease can be incredibly challenging. Often, prophylaxis treatment is given due to evidence that, during clinically quiescent times, subclinical viral transcription and translation may occur when an intact cell-mediated response is disrupted.2 Management becomes even more difficult since there is no consensus on exact dosing, frequency, duration or even the effectiveness of dosing.
The Zoster Eye Disease Study (ZEDS) is a prospective, multicenter, randomized control trial seeking to determine if prolonged suppressive antiviral therapy (Valtrex 1g daily) reduces anterior segment complications with HZO. In a survey conducted by the ZEDS investigators, over half of respondents reported using oral antivirals for a prolonged period for treatment of HZO. Long-term prophylactic antiviral treatment of acyclovir 400mg BID is often used to prevent reactivation of varicella zoster virus in the eye; however, this is not yet evidence-based. In this case, our patient was taking the valacyclovir dosage equivalent or higher than the recommended prophylactic treatment and still developed a recurrence.
Valacyclovir is the 1-valyl ester of acyclovir and can be rapidly converted to acyclovir. The bioavailability of valacyclovir is three- to five-times that of oral acyclovir. Valacyclovir, unlike acyclovir, can only be administered orally. Brand name valacyclovir, or Valtrex, is not recommended as the first-line therapy for immunocompromised patients due to its ability to have higher peak levels.3 The most common side effects of Valtrex are feelings of discouragement, sadness, irritability, as well as lack of appetite, loss of interest, tiredness and trouble sleeping. There have not been long-term studies on the Valtrex use for ocular herpes zoster, but there are many studies on its long-term use for genital herpes. In these, long-term use of valacyclovir (</=1,000mg per day) or acyclovir (800mg per day) was not associated with hematologic or clinical chemistry abnormalities. Valacyclovir use in immunocompromised patients can be more concerning due to possible liver function abnormalities.4 When choosing to prescribe oral antivirals (valacyclovir, acyclovir, etc.), renal impairment must be considered.
Another component in this case that we will have to manage long-term is the use of topical steroids. Our experience with this patient taught us that we cannot taper off topical prednisolone at this time, even if the patient appears quiescent and is on prophylactic orals. The patient has already had two slow tapers over the period of a month, in which his condition returned. Therefore, a lengthy conversation about the potential side effects of long-term topical use of steroids took place. The patient and providers agreed that the risks from topical steroids did not outweigh the potential loss of sight, and he will now only be tapered to BID until his cornea is clear for an extended period. During this time, we will continue to monitor his IOP and perform comprehensive exams.
The lessons learned from this patient were vast, starting with knowing there is not a standard protocol for managing patients once their initial HZO outbreak has healed. Literature evidence on taking oral Valtrex at higher dosages for extended periods of time has been proven safe when tested for other conditions; therefore, clinicians should be less eager to taper their patients off their oral antiviral quickly. Talking to patients about the side effects of long-term topical steroids and the risk of tapering them off too fast must be done at initial presentation. This way, both the patient and the doctor can feel confident in treatment until the virus has gone back into latency.
1. Hunt CM, Gregory HM, Gannon W. Oral valacyclovir treatment of herpes zoster ophthalmicus-induced optic neuritis. Cureus. 2021;13(8):e17033. 2. Cohrs RJ, Mehta SK, Schmid DS, Gilden DH, Pierson DL. Asymptomatic reactivation and shed of infectious varicella zoster virus in astronauts. J Med Virol. 2008;80(6):1116-22. 3. Tuft S. How to manage herpes zoster ophthalmicus. Community Eye Health. 2020;33(108):71-2. 4. Tyring SK, Baker D, Snowden W. Valacyclovir for herpes simplex virus infection: long-term safety and sustained efficacy after 20 years’ experience with acyclovir. J Infect Dis. 2002;186 Suppl 1:S40-6. |


