 |
 |
Lipid deposition can be a detrimental effect of many systemic pathologies. As healthcare providers, we often think about lipids depositing within arterial walls, presenting as lesions within cutaneous tissues or causing myriad vascular conditions such as carotid artery sclerosis and coronary artery disease. Its appearance in the eye, however, is less widely recognized. While we understand the importance of a systemic work-up for cases of lipemia retinalis or corneal arcus in young individuals, how do we address discovering dense lipid deposition within the cornea?
Lipid keratopathy (LK) is a disorder defined by abnormal deposition of lipids in the cornea. The lipids present as yellowish or opaque deposits within the corneal stroma (Figure 1A) and are usually associated with feeder vessels (Figure 1B). There are two main forms of LK—primary and secondary—which are discussed in further detail below.
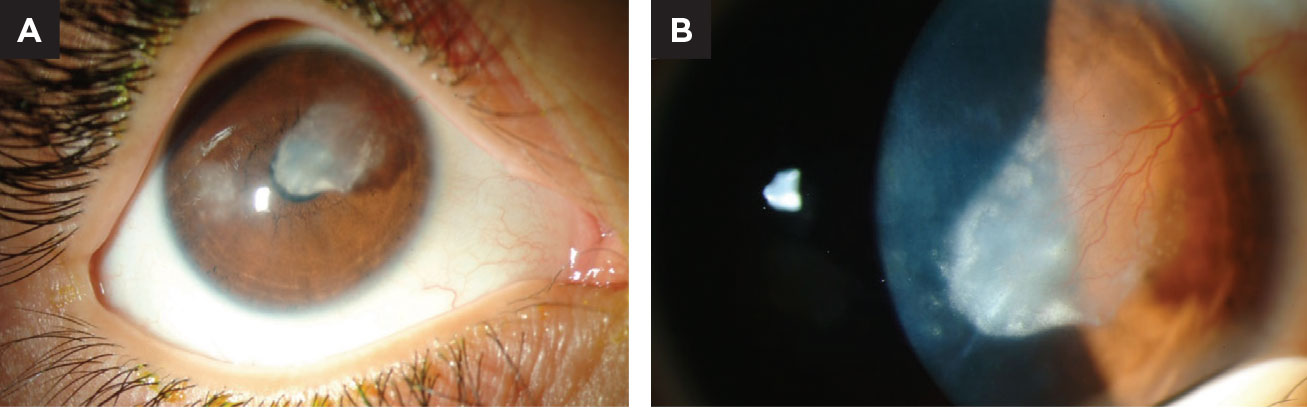 |
| Fig. 1. (A) This child has a history of bilateral herpes simplex keratitis (top). Notice the dense lipid deposition in the pupillary axis. (B) Closer magnification reveals a major feeder vessel is the cause of lipid exudation. Click image to enlarge. |
Primary Lipid Keratopathy
This form is extremely rare, occurring in the absence of any known systemic or corneal disorder. These cases tend to be bilateral in nature, and the lipid deposition is slowly progressive over a span of years. Often, the lipid is present full-thickness through the stroma and may even cause posterior bulging toward the anterior chamber.1 Histochemical analysis has found neutral fats, free fatty acids, phospholipids and cholesterol in affected eyes.2 Contrary to what one may anticipate, primary LK has not been associated with abnormal serum lipid profiles and remains largely idiopathic.3
Second Lipid Keratopathy
Nearly all the LK we see clinically is the secondary form. Though there are systemic causes for LK, such as lipoprotein metabolic disorder or Cogan’s syndrome, they are exceedingly rare. Secondary LK more commonly results from infectious keratitis, corneal trauma or surgery, interstitial keratitis or other forms of corneal inflammation. It can be found in non-inflammatory conditions such as Terrien’s marginal degeneration and has been associated with excessive soft or small-diameter rigid gas permeable contact lens wear.4,5 Corneal ulcers and herpetic keratitis are also commonly associated predecessors.
Corneal inflammation and subsequent neovascularization (NV) precede LK in most cases. Once blood vessels and lymphatic channels invade the avascular cornea, they act as mini freeways by which blood constituents gain access to the stroma. The lipid deposits may present at any depth but typically exist at the terminus or along the path of the vascular changes.
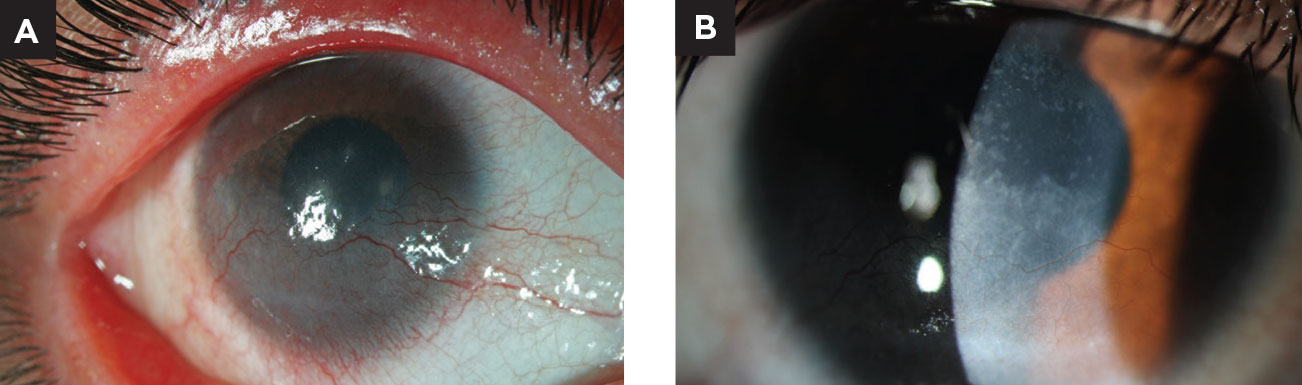 |
| Fig. 2. This young patient presented with superficial corneal NV due to chronic blepharitis. She was treated with lid hygiene, oral antibiotics and topical corticosteroids before transitioning to maintenance therapy of cyclosporine, compounded topical doxycycline and scleral contact lens wear. Note the improvement in the superficial vascularization. Photos courtesy of Stephanie Frankel, OD. Click image to enlarge. |
Management
Depending on the area of involved cornea, patients’ complaints can range from being completely asymptomatic to experiencing debilitating loss of visual function. Eliminating the stressor—such as excessive contact lens wear, for example—should be our first step. Herpetic keratitis is among the most frequent culprits. In these settings, it is prudent to consider long-term prophylactic dosage of oral antivirals and recommend avoidance of possible reactivation triggers, such as excessive ultraviolet exposure or significant stress.
Once a visually significant lipid is present within the stroma, we should consider additional strategies. The pathologic neovascular vessels have been a popular treatment target due to their role in LK pathogenesis. Topical steroids have a positive impact in prevention of NV, but can cause small vessel regression.6 Though steroids should be considered a first-line option due to their ease of accessibility and relatively low-risk, their effectiveness may be limited with large-caliber vessels or NV that is not primarily inflammatory in nature. Superficial vascularization from inflammatory causes can also respond favorably to topical cyclosporine or compounded doxycycline, but these topical treatments will likely fall short in cases of deep stromal NV, which is more commonly responsible for LK (Figure 2).
When readily available treatments fail to improve a patient’s LK and deep corneal NV, the management may need to be escalated. Some treatment approaches center on directly obstructing the vessel arborization. A few of these tactics include photodynamic therapy (PDT) and photocoagulation.
PDT works by first administering a vascular-selective, photosensitizing agent either intravenously or topically. Then, the area of NV is irradiated with activating light, leading to vascular endothelial damage and microvascular occlusions in the aberrant blood vessels.7 This treatment can be repeated and is minimally invasive, but it is relatively expensive and risks include possible stromal scarring and inadvertent retinal irradiation.8
Argon laser photocoagulation has also been used with success, but concerns arise regarding vessel recanalization, iris atrophy, descemetocele formation and risk of anflammatory response to the thermal damage.8,9
Fine-needle diathermy is an additional means of direct vascular occlusion, targeted at large, arborized vessels. A single needle is used in conjunction with an unipolar diathermy unit to directly treat each individual feeder vessel. Results have proven largely successful in causing regression of NV, but the treatment may need to be repeated in a select group of patients. The main risks are intrastromal hemorrhage (which clears with time), corneal microperforations and epithelial defects.10,11 Once the vessels are successfully occluded and any comorbid inflammation is managed, LK may gradually resolve.
Antiangiogenic therapies are among the newest class of treatments that focus on reducing vasculature within the stroma. Promoters of corneal NV include VEGF and other growth factors, matrix metalloproteinases and pro-inflammatory cytokines. Anti-VEGF agents dominate retina therapy, and have also proven beneficial for use in the anterior segment realm, as they reduce the formation of blood vessels and lymphatic channels.12 They have been used as topical ophthalmic drops as well as intrastromal and subconjunctival injections.
While all preparations have shown reduction in corneal NV and even lipids in some cases, one study noted spontaneous epithelial defect and stromal thinning with application of the topical drop.13 This could signal that VEGF maintains a delicate role in wound healing and maintaining epithelial integrity, but overall it remains a promising avenue for corneal NV and LK.
Summary
Though relatively uncommon, LK can significantly impact patients and we should be aggressive in treating early inflammatory precursors. If prevention isn’t possible, various treatments can help clear the cornea and improve visual outcomes.
1. Silva-Araujo A, Tavares MA, Lemos MM, et al. Primary lipid keratopathy: a morphological and biochemical assessment. Br J Ophthalmol, 1993. 77(4): 248-50. 2. Alfonso E, Arrellanes L, Boruchoff SA, et al. Idiopathic bilateral lipid keratopathy. Br J Ophthalmol, 1988. 72(5): 338-43. 3. Hall MN, Moshirfar M, et al., Lipid keratopathy: a review of pathophysiology, differential diagnosis, and management. Ophthalmol Ther, 2020. 9(4): 833-852. 4. Cressey A, Jacobs DS, et al. Management of vascularized limbal keratitis with prosthetic replacement of the ocular surface system. Eye Contact Lens, 2012. 38(2): 137-40. 5. Braude LS, Sugar J. Circinate-pattern interstitial keratopathy in daily wear soft contact lens wearers. Arch Ophthalmol, 1985. 103(11): 1662-65. 6. Hos D, Ssaban DR, Bock F, et al. Suppression of inflammatory corneal lymphangiogenesis by application of topical corticosteroids. Arch Ophthalmol, 2011. 129(4): 445-52. 7. Igarashi T, Takahashi H. Photodynamic therapy for neovascularization in lipid keratopathy. J Nippon Med Sch, 2010. 77(2): 66. 8. Corrent G, Roussel TJ, et al. Promotion of graft survival by photothrombotic occlusion of corneal neovascularization. Arch Ophthalmol, 1989. 107(10): 1501-06. 9. Marsh RJ Argon laser treatment of lipid keratopathy. Br J Ophthalmol, 1988. 72(12): 900-04. 10. Pillai, CT, Dua HS, PHossain P. Fine needle diathermy occlusion of corneal vessels. Invest Ophthalmol Vis Sci, 2000. 41(8): 2148-53. 11. Faraj LA, Elalfy MS, Said DG, et al. Fine needle diathermy occlusion of corneal vessels. Br J Ophthalmol, 2014. 98(9): 1287-90. 12. Hsu CC, Chang HM, Lin TC, et al. Corneal neovascularization and contemporary antiangiogenic therapeutics. J Chin Med Assoc, 2015. 78(6): 323-30. 13. Kim SW, Ha BJ, Kim EK, et al. The effect of topical bevacizumab on corneal neovascularization. Ophthalmology, 2008. 115(6): e33-38. |


