 |
A 58-year-old Caucasian male presented to the emergency room with worsening vision loss and right eye pain over the past three days. Prior to examination, the patient reported poor compliance with his contact lenses and admitted to sleeping in them for extended periods of time. The patient reported being seen by an outside provider four days prior who had diagnosed him with a corneal abrasion. A bandage contact lens was placed and he was started on ofloxacin q2h. He reported not starting the ofloxacin for two days after that visit and had worsening vision with ofloxacin once started, which prompted his visit to the emergency room. The patient’s systemic history was non-contributory.
Entrance testing was performed with the following results: visual acuity was measured at counting fingers OD and 20/25 OS with prescription glasses; pupils were ERRLA without afferent pupillary defect OU; confrontation visual fields were full to finger counting OU. Slit lamp examination was performed showing a near complete corneal epithelial defect with large superior arcuate infiltrate measuring 5mm horizontal by 3mm vertical OD, mucopurulent/suppurative discharge OD and 3+ anterior chamber reaction with 2mm hypopyon OD. The slit lamp exam OS was unremarkable. Intraocular pressure (IOP) was measured with Tono-Pen Avia (Reichert), reading 45mm Hg OD and 12mm Hg OS. The patient was dilated OD with two drops of 1% cyclopentolate in-office. A dilated fundus exam was attempted OD, but the fundus was unable to be appreciated. A B-scan was performed OD showing no anterior chamber spillover, vitritis, retinal masses or detachment.
There was strong suspicion for Pseudomonas due to the characteristic soupy purulence and fibrinous exudate; however, culturing was ordered for confirmation. The patient was started on the following medications: fortified vancomycin 25mg/ml q1h and fortified tobramycin 15mg/ml q1h alternating every half hour OD, dorzolamide-timolol 22.3mg-6.8mg/ml twice a day OD, doxycycline hyclate 100mg twice a day and vitamin C 1000mg. He was instructed to follow up the next day with the outpatient clinic.
Testing results were returned the following day with gram staining showing the presence of a gram-negative rod bacteria suggestive of Pseudomonas. Culturing results confirmed the presence of over 100 colonies of Pseudomonas aeruginosa which was susceptible to amikacin, cefepime, ciprofloxacin, meropenem, piperacillin/tazobactam and tobramycin.
The patient did not return the following day, instead electing to follow up with his local ophthalmologist, but ultimately returned to the clinic six weeks later following his visit to the emergency room. He was noted to have non-responding elevated IOP of 45mm Hg OD with an increasingly shallow anterior chamber. A slit lamp exam showed a 5mmx5mm non-healing central epithelial defect without infiltration, significant corneal scarring and neovascularization. At this time, it was decided he be scheduled for an Ahmed glaucoma valve (New World Medical) with scleral patch graft, penetrating keratoplasty and possible cataract extraction with intraocular lens implantation, anterior vitrectomy and anterior chamber reconstruction.
Throughout the next few months, the patient experienced several complications, including scleral melt with scleral abscess exploration and intravitreal injection, corneal melt with Tutoplast pericardium allograft (Coloplast) and amniotic membrane. Following this string of complications, the patient was scheduled and underwent surgery for implantation of the Boston aphakic keratoprosthesis Type I (KPro; Massachusetts Eye and Ear Infirmary).
 |
|
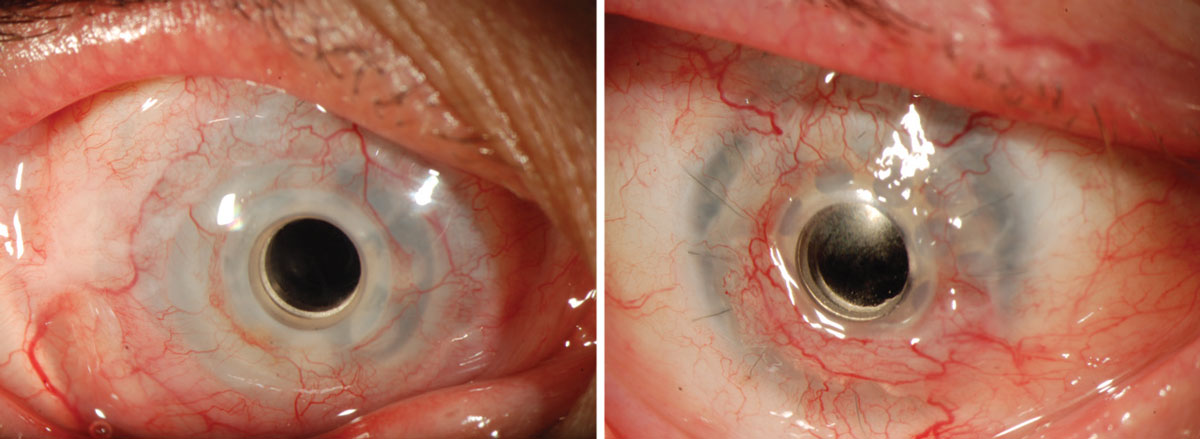
The KPro prosthesis as it looks in the eye. Click image to enlarge. |
KPro Characteristics
Over the past 30 years, the Boston KPro Type 1 has become an increasingly popular option for patients with significant ocular pathology. Indications include Stevens-Johnson syndrome, ocular cicatricial pemphigoid, autoimmune etiologies, ocular burns and severe graft-vs.-host disease.1,2 Consequently, optometrists are becoming more likely to see this once-rare ocular prosthesis in their exam lanes. It is important to understand the device design, clinical indications and visual outcomes for when it does appear.
The KPro contains three main components: a front plate, a corneal graft button and a back plate. The former is made of polymethylmethacrylate (PMMA). This plate contains an optical stem which allows light to the retina. The back portion of this stem contains a locking interface which allows the back plate to be attached. Additionally, the back plate can be ordered in either PMMA or titanium. Donor tissue lies between the front and back plates. Throughout the years, advances have been made to the original Boston KPro design, including the addition of holes in the back plate which allows for the diffusion of aqueous to support the donor graft stroma and keratocytes. It is this back plate containing multiple fenestrations that gives the KPro its characteristic appearance. Those of us who fit scleral lenses can think of this similarly to a scleral lens modality. Later, the titanium locking c-ring was altered to create a threadless assembly of the Boston KPro. The most recent advancement was the addition of a titanium back that is believed to improve biocompatibility and retention of fluid.3
Initially, the KPro was designed and approved in the usage for severe corneal disease and multiple corneal transplant failures. The approved indications have steadily increased to include conditions like advanced limbal stem cell deficiency and even eyes which have undergone silicone oil implantation. At the time of this article’s publication, the most common indication remains the usage following multiple corneal transplant failures. Studies continue to show the survival rate of penetrating keratoplasties declines with each successive surgery. One study showed the survival rate of secondary grafts at two and five years to be 64% and 46%, respectively, compared to primary grafts in a Canadian study which showed survival rates of 79% and 65%.4,5
Recent studies have shown that using the KPro as an initial procedure instead of corneal transplantation followed by KPro produced better visual outcomes.6-8 The majority of patients who underwent implantation showed an improvement in best-corrected visual acuity (BCVA) better than 20/200. Underlying cause for surgery appears to play a role in visual outcome as well. For example, of patients with chemical injury causes, approximately 65% reached BCVA greater than 20/200 compared with only 25% of patients with herpes zoster as the cause.9,10 The majority of KPro recipients are of adult age, as pediatric patients tend to have a lower device retention rate, but studies and patient populations are limited.11
The Boston KPro exists in more than one type—Type I and Type II. The Type II KPro is usually reserved for extreme cases and is done with an accompanying permanent tarsorrhaphy.
With knowledge of the basic components of the KPro, it is important to know what else to look for if one is in your chair. Commonly, these patients are also outfitted in large-diameter soft contact lenses. These bandage contact lenses are necessary for KPro patients to maintain adequate corneal graft hydration while minimizing the risk of complications.12,13 My next column will focus on what type of bandage contact lenses are used for Boston KPro and how to examine them for proper fit and complications.
1. Doane MG, Dohlman CH, Bearse G. Fabrication of a keratoprosthesis. Cornea. 1996;15(2):179-84. 2. Shihadeh WA, Mohidat HM. Outcomes of the Boston keratoprosthesis in Jordan. Middle East Afr J Ophthalmol. 2012;19(1):97-100. 3. Khan BF, Harissi-Dagher M, Khan DM, Dohlman CH. Advances in Boston keratoprosthesis: enhancing retention and prevention of infection and inflammation. Int Ophthalmol Clin. 2007;47(2):61-71. 4. Weisbrod DJ, Sit M, Naor J, Slomovic AR. Outcomes of repeat penetrating keratoplasty and risk factors for graft failure. Cornea. 2003;22(5):429-34. 5. Sit M, Weisbrod DJ, Naor J, Slomovic AR. Corneal graft outcome study. Cornea. 2001;20(2):129-33. 6. Driver TH, Aravena C, Duong HNV, et al. Outcomes of the Boston type I keratoprosthesis as the primary penetrating corneal procedure. Cornea. 2018;37(11):1400-7. 7. Fadous R, Levallois-Gignac S, Vaillancourt L, Robert MC, Harissi-Dagher M. The Boston keratoprosthesis type 1 as primary penetrating corneal procedure. Br J Ophthalmol. 2015;99(12):1664-8. 8. Kang KB, Karas FI, Rai R, et al. Five year outcomes of Boston type I keratoprosthesis as primary versus secondary penetrating corneal procedure in a matched case control study. PLoS One. 2018;13(2):e0192381. 9. Shanbhag SS, Saeed HN, Paschalis EI, Chodosh J. Boston keratoprosthesis type 1 for limbal stem cell deficiency after severe chemical corneal injury: a systematic review. Ocul Surf. 2018;16(3):272-81. 10. Brown CR, Wagoner MD, Welder JD, et al. Boston keratoprosthesis type 1 for herpes simplex and herpes zoster keratopathy. Cornea. 2014;33(8):801-5. 11. Fung SSM, Jabbour S, Harissi-Dagher M, et al. Visual outcomes and complications of type I Boston keratoprosthesis in children: a retrospective multicenter study and literature review. Ophthalmology. 2018;125(2):153-60. 12. Ament JD, Spurr-Michaud SJ, Dohlman CH, Gipson IK. The Boston keratoprosthesis: comparing corneal epithelial cell compatibility with titanium and PMMA. Cornea. 2009;28(7):808-11. 13. Cherny C, Sherman S, Trief D. Contact lens modifications for Boston keratoprosthesis: novel case report and review of the literature. JCLRS. 2022;6(1):e9-17. |


