  |
A 65-year-old female presented due to severe pain, redness and blurred vision in her right eye. Ocular history was significant for cataract surgery OU and pterygium excision OS. She did not have a history of autoimmune disease.
Prior to the patient’s visit to our office, another eye care provider had noted scleral thinning and inflammation OD. This was thought to have been caused by an eroding scleral hyaline plaque, which was then manipulated but not removed. Over the subsequent month, her condition worsened and she developed conjunctival breakdown, pain, blurred vision, adjacent corneal thinning and a small hypopyon. At that point, she was referred to our ophthalmic emergency department.
Examination
At presentation, the right eye showed severe diffuse injection, an exposed calcific plaque with underlying scleral thinning and an adjacent abscess (Figure 1). Vision had decreased from a previously documented measurement of 20/25 to 20/100.
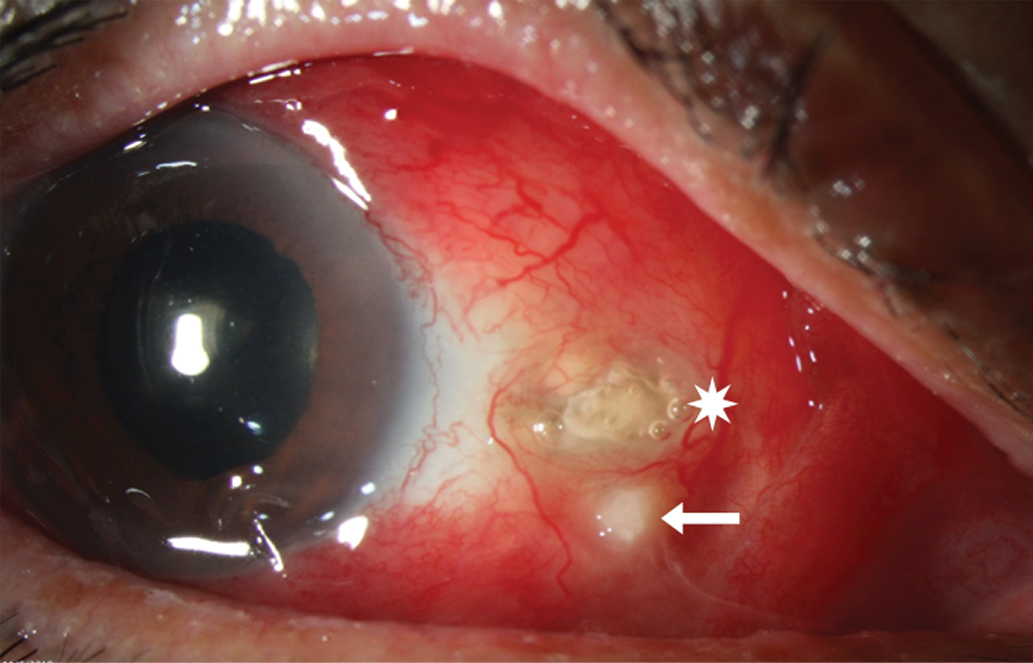 |
Fig. 1. Intense scleral injection with focal necrosis associated with eroded calcific plaque (white star) and scleral abscess (white arrow). Click image to enlarge. |
Visualization of the posterior segment was limited due to incomplete pupillary dilation. An ultrasound revealed diffuse choroidal thickening, shallow 360° anterior choroidal detachment, an inferior serous retinal detachment and Tenon’s infiltration nasally. The vitreous cavity was clear, ruling out vitritis (Figure 2). Clinical features suggested an infectious component, so a cornea surgeon obtained a scleral culture.
The patient was placed on fortified vancomycin, fortified tobramycin, oral moxifloxacin, oral vitamin C and a topical cycloplegic agent. Corticosteroids were withheld until inflammatory and infectious lab results became available. The blood test results were non-contributory, but the scleral culture revealed the diagnosis: infectious scleritis secondary to Pseudomonas aeruginosa.
Discussion
The word “scleritis” likely conjures up a clinical picture of a deeply inflamed sclera with boring pain. It may be accompanied by headache, photophobia and even vision loss. Scleritis is an umbrella term often defined by location (anterior vs. posterior) or clinical features (e.g., nodular, diffuse, necrotizing). An additional way we can identify scleritis, which may be overlooked due to its rarity, is by the presence or absence of an infection.
The distinction between infectious and non-infectious scleritis carries significant weight from an early point in the management process. Immune-mediated scleritis is typically less severe and more common than its infectious counterpart, making up approximately 90% to 95% of all cases of scleritis.1 Non-infectious scleritis may be idiopathic, but up to 50% of cases are associated with an underlying autoimmune disorder or connective tissue disease, such as rheumatoid arthritis, inflammatory bowel disease, granulomatosis with polyangiitis or relapsing polychondritis. Infectious scleritis, on the other hand, has unique risk factors, clinical features and treatment protocols.
Infectious scleritis presents clinically with significant redness, tearing, pain and often an anterior chamber reaction. Suppurative discharge and the presence of a scleral ulcer or abscess, which appears as a white or yellowish nodule under the conjunctiva, should raise suspicion. In some cases, there may be an associated calcific plaque.2
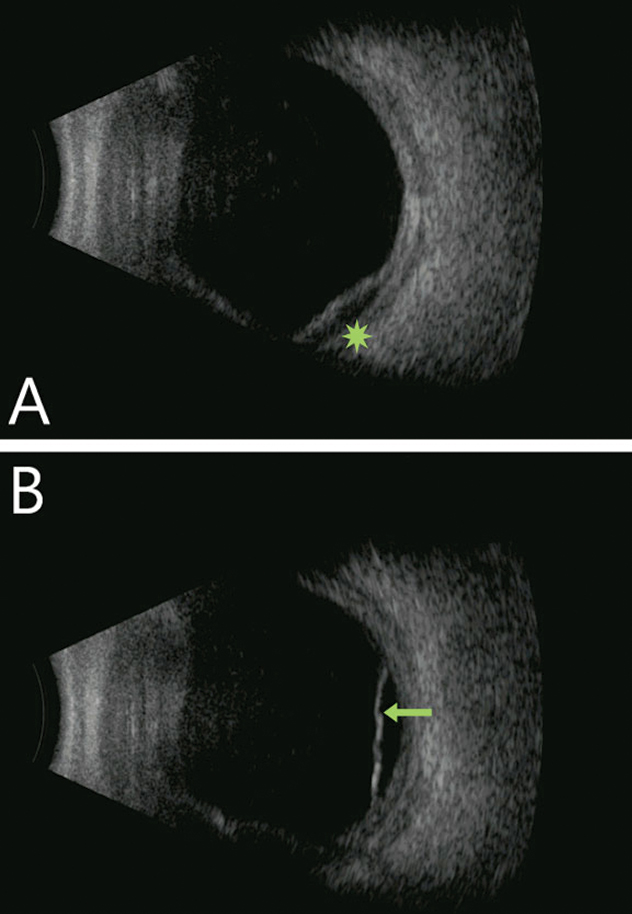 |
| Fig. 2. Diffuse choroidal thickening with focal choroidal detachment (green star) and with shallow serous retinal detachment (green arrow). Notably, the vitreous cavity is quiet in both images. Click image to enlarge. |
In suspected infectious scleritis, the importance of a complete ophthalmic history cannot be overstated, as it often reveals prior ocular trauma or surgery. A retrospective review found that the shortest interval between an inciting event and the onset of infectious scleritis occurred in cases of ophthalmic trauma, in which symptoms appeared between zero and three months (average of 0.2 months).3 Retina, glaucoma and cataract surgeries were associated with a slightly longer interval (1.0 to 1.6 months).3 The period before symptom onset was considerably longer in pterygium surgery, averaging 49 months (range of zero to 183 months), but it has been documented up to four decades later.3,4
Among the procedures implicated as risk factors for developing infectious scleritis, the most common is pterygium excision.3 This surgery causes scleral thinning and is often coupled with antimetabolites, such as mitomycin C, or adjunctive therapies, including beta irradiation, aggressive vessel cautery and bare sclera techniques. These can compromise episcleral vascular supply and lead to inadequate wound healing and avascular necrosis, thereby providing a nidus for microbial adherence.5
Obtaining a scleral culture is critical in infectious scleritis. Bacterial strains, specifically Pseudomonas species, are by far the most common causative organism of infectious scleritis in developed countries.1,3,5-8 A study conducted in India revealed the most commonly implicated pathogens in the country’s population were fungi (38%) and filamentous bacteria (Nocardia: 24%).1 This suggests humid climates and agricultural societies may be at higher risk for these atypical forms of infection.1
Fortified antibiotics are typically initiated at presentation, but subsequent use is guided by lab findings. Due to the avascular and densely packed nature of scleral tissue, however, antibiotic treatment alone is often insufficient. Oral antibiotics have proven to be an effective treatment for bacterial scleritis, and potent anti-inflammatory agents, such as corticosteroids, may be judiciously added after appropriate antimicrobial treatment has been initiated.8 Severe infectious scleritis often necessitates surgical interventions, such as glued or sutured amniotic membranes and debridement of necrotic tissue. These methods are employed to decrease inflammatory response and increase drug penetration.
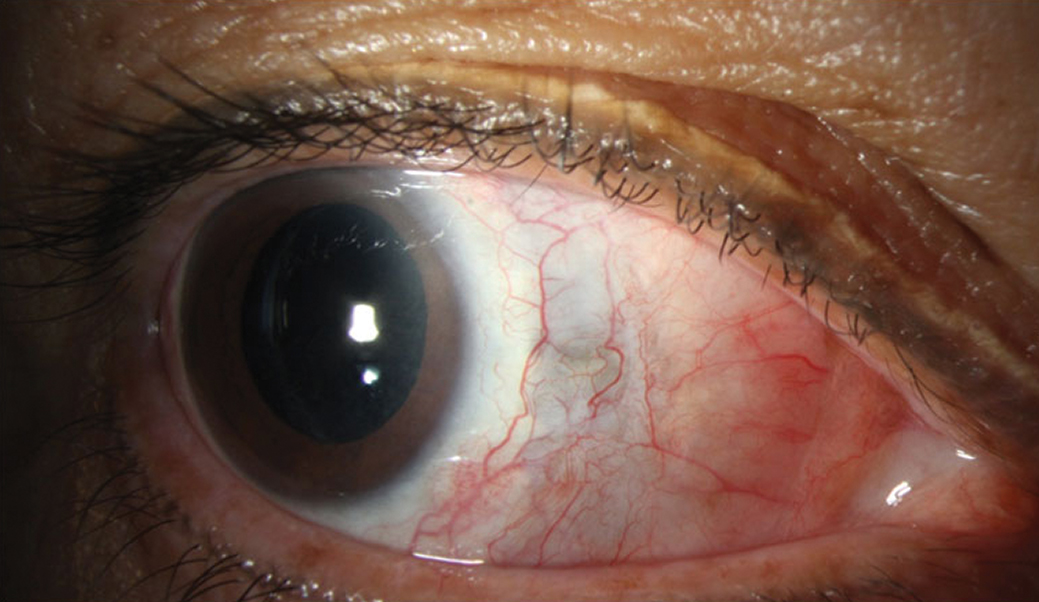 |
| Fig. 3. Improvement in injection and resolution of abscess. Residual scleral thinning is evident by the bluish hue. Click image to enlarge. |
Negative long-term effects of infectious scleritis are common. Scleral perforation, glaucoma, retinal detachment, cataract and endophthalmitis are all potential complications. Enucleation is ultimately required in 4% to 33% of these cases.3,8,9 Unfortunately, even with prompt treatment and infection resolution, vision is often compromised, with worse presenting visual acuity translating to poorer visual outcomes.
Case Outcome
Our patient returned for frequent follow-up. One week after presenting, she underwent necrotic scleral tissue debridement with antibiotic washout and amniotic membrane graft placement. She later received a sub-Tenon’s triamcinolone injection and was maintained on a combination of topical and oral antibiotics, topical and oral corticosteroids, vitamin C, doxycycline and topical cycloplegia. In the following months, she developed diplopia as a complication of secondary myositis, but spontaneous resolution of the choroidal and serous retinal detachments was noted.
Six months into treatment, her pain resolved and her visual acuity stabilized at 20/200. Her extraocular motilities, though mildly restricted, continue to be followed (Figure 3).
Despite the rarity of infectious scleritis, it is important to be aware of this disease and its complications. Early recognition, effective treatment and appropriate surgical consult are integral to preserving vision and, in severe cases, the eye.
1. Jain V, Garg P, Sharma S. Microbial scleritis-experience from a developing country. Eye (Lond). 2009;23(2):255-61. 2. Ramenaden ER, Raiji VR. Clinical characteristics and visual outcomes in infectious scleritis: a review. Clin Ophthalmol. 2013;7:2113-22. 3. Hodson KL, Galor A, Karp CL, et al. Epidemiology and visual outcomes in patients with infectious scleritis. Cornea. 2013;32(4):466-72. 4. Su CY, Tsai JJ, Chang YC, et al. Immunologic and clinical manifestations of infectious scleritis after pterygium excision. Cornea. 2006;25(6):663-6. 5. Cunningham MA, Alexander JK, Matoba AY, et al. Management and outcome of microbial anterior scleritis. Cornea. 2011;30(9):1020-3. 6. Paula JS, Simão MLH, Rocha EM, et al. Atypical pneumococcal scleritis after pterygium excision: case report and literature review. Cornea. 2006;25(1):115-7. 7. Altman AJ, Cohen EJ, Berger ST, et al. Scleritis and Streptococcus pneumoniae. Cornea. 1991;10(4):341-5. 8. Ahmad S, Lopez M, Attala M, et al. Interventions and outcomes in patients with infectious Pseudomonas scleritis: a 10-year perspective. Ocul Immunol Inflamm. 2019;27(3):499-506. 9. Reynolds MG, Alfonso E. Treatment of infectious scleritis and keratoscleritis. Am J Ophthalmol. 1991;112(5):543-7. |


